Learning Objectives
This is an intermediate-level course. After completing this course,
mental health professionals will be able to:
- Discuss ethical and
legal considerations in providing information about medications to clients.
- Explain medication
treatment algorithms for depressive disorders, anxiety disorders, ADHD, and
bipolar spectrum disorders.
- Troubleshoot treatment-resistant
cases of depression.
- List experimental
treatments for treatment-resistant mental illness and the data supporting them.
- Describe the newest
treatments for postpartum depression.
The materials in this course are based on the most accurate
information available to the author at the time of writing and this course has been updated by Li Liang, MD. New developments in
the field of psychopharmacology occur each day and new research findings may
emerge that supersede these course materials. This course is updated regularly
as new practice guidelines are developed. This course will equip clinicians to
evaluate the needs for medical treatment for their psychotherapy clients, to
assess responses to treatment and to more effectively collaborate with primary
care physicians and psychiatrists.
Outline
- Introduction
- Preliminary
Considerations
- Legal and Ethical Issues
- Drug Metabolism and Medication Dosing
- The Process of Approving a New Drug by the FDA
- Depression
- Introduction
- A Changing Treatment Landscape
- Treatment Outcomes
- Abnormal Neurobiology: A Compelling Case for the Use of Antidepressants
- Diagnostic Issues
- Psychopharmacology of Depression
- Antidepressant Medications
- Pharmacologic Treatment Guidelines
- First-Line Medication Choices: Major Depression
- Major Depressive Disorder (MDD) Treatment
Algorithm: Choosing a First-Line Antidepressant
- Partial and Non-Responder Strategies
- Phases of Treatment for Major Depressive Disorder
- Treatment for Other Types of Depression
- New Treatments and Interventional Treatment for Major
Depressive Disorder
- Herbs and Supplements for Major Depressive Disorder
- Combined Psychotherapy and Pharmacotherapy
- Bipolar Spectrum
Disorders
- Introduction and Diagnostic Issues
- Symptoms of Classic Mania
- Symptoms of Hypomania
- Symptoms of Mania or Hypomania with Mixed
Features
- Treatments for Bipolar Disorder
- Lifestyle Management
- Medication Treatments: General Considerations
- Medication Treatments: What are Realistic
Outcomes?
- Bipolar Medications
- Other Medications We Can Use to Treat Bipolar
Spectrum Disorder
- Targets for Medication Treatment
- Getting Started with Medication Treatment:
Emergency Medication Treatments and Laboratory Tests
- Treatment Guidelines: Mania
- Treatment Guidelines: Bipolar Depression
- Adjunctive Therapies for Bipolar Spectrum
Disorder
- Anxiety Disorders
- General Considerations
- Diagnostic Issues
- Psychopharmacology For Anxiety Disorders
- Generalized Anxiety Disorder (GAD)
- Panic Disorder
- Social Anxiety
- Herbs and over-the-counter supplements
- Attention
Deficit/Hyperactivity Disorder (ADHD)
- Introduction
- Diagnostic Issues
- Neurobiology
- Psychopharmacology of ADHD
- Appendix
- I. Psychiatric Medications: Quick Reference Guide
- II. Mood Stabilizers/Anti-manic Agents
- III. Miscellaneous
- IV. Caffeine Questionnaire
- Suggested Readings/Resources
- References
Note: “Quick Reference to Psychiatric Medications” is
available as a free download on the following website. It is typically updated
once or twice a year. It may be helpful to have a copy of this as you read the
following material: apadivisions.org/division-55/councils/research/quick-reference.pdf.
Introduction
This course is designed to teach the
basics of clinical psychopharmacology for practicing psychotherapists. The
focus will be on three groups of commonly encountered clinical conditions: mood
disorders (depression and bipolar spectrum disorders), anxiety disorders, and
ADHD. An assumption is made that you are very familiar with psychopathology and
DSM-5-TR diagnostic criteria, however there will be supplemental diagnostic
information presented in this course as it applies to treatment decision-making.
More recent epidemiological studies and new findings in the neurosciences have
influenced changes in some diagnostic criteria in the DSM-5-TR which will be
addressed in this course. In addition, there will be brief mention of
neurobiology and pathophysiology associated with certain clinical conditions,
although a comprehensive discussion of these topics is beyond the scope of this
course (see J. Cooper, et al., 2003 and Preston, et al., 2017 for a more
detailed review). The primary focus of this course is to provide a current
overview of psychopharmacologic treatment guidelines. Many of these are derived
from large-scale empirical studies (often referred to as algorithm studies).
Treatment guidelines do not represent personal opinions of the author, but
rather are presentations of algorithms that have been developed by NIMH-supported
programs, guidelines from the American Psychiatric Association, and the Texas
Medication Algorithm Project.
This is the first course in a three-course series. This first course teaches the basics of psychopharmacology. The second course, Bipolar Spectrum Disorders: Diagnosis and Pharmacologic Treatment, focuses on understanding common bipolar spectrum disorders and recognizing symptoms of mania, symptoms of hypomania, and symptoms of depression. The third course, Psychopharmacology for Child and Adolescent Mood Disorders: Problems and Promise, covers mood disorders in youth, including using psychotropic medication to treat youth.
All psychotherapists must be familiar with psychopharmacology for
three reasons:
- It is important to
know which classes of medication we use to treat different disorders so that
referrals can be made to an appropriate prescriber. It is a part of informed
consent to provide clients with information regarding all viable treatment
options for their particular disorder so that they can make choices about which
treatment to pursue.
- It is important to be
able to communicate clearly with physicians to whom our clients are referred to
share relevant information about the diagnosis and the client’s response to
treatment.
- In the current managed
care practice model, increasing numbers of clients are receiving psychiatric
medication treatment in their primary care physician’s office. Here the amount
of time spent with the physician may be very limited. Our clients often want
and need additional information regarding their medication treatment that is
not readily available from the primary care doctor. Psychotherapists can be
very instrumental in providing important information regarding such issues as:
side effects, how much time is required for medications to begin working,
realistic expectations for treatment outcomes with medications, etc.
Preliminary Considerations
Legal and Ethical Issues
A review of case law reveals a number of cases in which
non-physician health care professionals have been accused of practicing
medicine without a license because they gave patients information regarding
their medications and medical treatment. Obviously, it is important for all
therapists to practice within their scope of practice, to do whatever is in the
best interests of our patients and to be on solid ethical ground. It is clear
from the existing case law that it is unethical and illegal to tell patients
either: 1. to stop taking a medication, or 2. to change the dose of a
medication. This is considered practicing medicine. However, in every case
(with one exception addressed below) those who provided information regarding
medicines and medication treatment and were accused of practicing medicine
without a license were found to be not guilty. Further, in half of the cases, the
judge said that to not provide information to patients may have been acting in
a professionally incompetent manner.
Here is what this is based on. First and foremost is the right
granted by the first amendment, the right to free speech. Secondly, the
following are deemed appropriate to share with patients, if and only if the
therapist has training and is knowledgeable regarding the facts of medication
treatments:
- Once the diagnosis is
made, telling the patient about treatment options. This is a part of “informed
consent” in which the therapist must share with patients their available
treatment options – mentioning those treatments that are empirically supported.
Therefore, even if a therapist is not especially fond of pharmacological
treatments, it behooves them to share information regarding drug treatments. At
the heart of informed consent is a respect for our clients in terms of allowing
them to be the final judge in opting for certain treatments.
- Discussion about
medication side effects.
- Discussion regarding
the likely or common positive benefits of drug treatments (i.e., what kinds of
symptoms are likely to be improved with medications).
- Discussion regarding
the length of time that it may take for medications to begin to show clinical
effects.
- Limitations of
medication treatments.
- Underscoring the
importance of collaborative treatment, that is, the value in having both the
therapist and the prescribing doctor share information about the course of
treatment. For example, this communication might include amount of improvement, lack of improvement, emergent side effects.
- There is one case in
which a non-medical clinician was found to be unethical and this was due to the
fact that this person only recommended an over-the-counter product but failed
to talk about other available treatment options. As will be discussed below,
some over-the-counter products do have empirical support for efficacy in
treating some psychiatric disorders, and can be mentioned to patients as
options. However, this must be done in the context of presenting all available
empirically validated treatment options.
Due to the impact of managed care practice, as mentioned earlier, many patients are
receiving prescriptions for psychotropic medications from primary care
physicians who may have very limited time to spend with the patients both initially,
when the diagnosis is made and treatment is initiated, and in follow-up. This
is a significant problem and psychotherapists can provide enormous help to
patients by monitoring their response to medication and providing
support and information regarding drug treatments, and they should be able to
do so in ethical and legal ways. Ultimately, the care of our patients is
paramount.
Drug Metabolism and Medication Dosing
For each psychiatric medication discussed in this text, you will
see listed the typical adult daily doses. In many instances the “therapeutic
dosage range” is broad. For example, daily dosing with lithium is between 600
and 2,400 mg. or for Prozac, 10 to 80 mg. per day. It is important to know that
the amount of medication required to effectively reduce and eliminate symptoms
often has little to do with how severe the symptoms are. And what matters is
not so much how much drug is ingested but rather, how much of the medication
enters the blood stream.
There are three primary factors that influence the amount of drug
that finds its way into the blood stream. First is the rate of liver
metabolism. Psychotropic medications are absorbed through the walls of the
stomach and intestines and go directly to the liver. Here the drug molecules
are acted upon by liver enzymes that begin a process generally referred to as
biotransformation. Liver enzymes chemically alter the medication in ways that either
activate the medication or allow the drug to be more readily excreted from the
body. The liver’s function is to detoxify the body. Thus, in this so-called
“first pass effect” through the liver, a good deal of the drug is chemically
transformed and then rapidly excreted from the body. However, some of the
medication initially escapes this process and makes its way through the liver
and into circulation, and thus is allowed to begin accumulating in the bloodstream.
How rapidly the liver metabolizes drugs depends on a number of factors. This
resulting blood level is what matters when it comes to reducing symptoms.
(Note: two psychotropic medications are not metabolized in the liver: lithium
and Neurontin).
Genes play a significant role in this process. A small percentage
of people are known as rapid metabolizers. They take certain drugs and then
eliminate them very quickly. The result is that even though they may be taking
what seems like an adequate dose of the medication, little actually gets into
the blood stream. Once it is discovered that someone is a rapid metabolizer, it
is usually required that they be prescribed higher doses of medications and
eventually enough gets into the bloodstream to be effective. Again, this has
nothing to do with how severely ill they are … it’s just a matter of the
liver’s metabolic rate.
Converse to this are the hypo-metabolizers. This also small
percentage of people (perhaps 5% of the general population) have fewer
than average liver enzymes. It is important to note that those of Asian and
African descent show higher percentages of hypo-metabolism, approximately 33%,
and thus more caution should be taken with initial dosing in these individuals
to avoid significant side effects. The effect is that they can take a “typical”
starting dose of a medication, and on its trip through the liver, only small
amounts are transformed and excreted. The result is often very high blood
levels of the medication and severe side effects or toxicity. Thus the ultimate
solution for hypo-metabolizers is to use very small doses of medications
initially and then increase doses gradually. Sometimes when a person is first treated,
they will experience serious side effects and this may be due to
hypo-metabolizing. It is often hard to know ahead of time if this will happen
with any one given individual. Thus, if your patient has had an experience of
encountering very intense side effects with other medications in the past, one
may anticipate that they may be a hypo-metabolizer, and thus initial dosing
should be low and increased dosing should be done gradually.
A second factor determining blood levels of medications is the
functioning of the kidney. Sometimes genetic factors play a role here too, but
more often problems can occur due to kidney disease. Thus, for some bipolar
medications in particular, pretreatment laboratory testing will include an
assessment of kidney functioning. This is especially important for patients
being treated with lithium; it eliminates without metabolism and is excreted by
the kidney.
Finally, a number of drugs can adversely affect liver metabolism
and thus alter blood levels. Here is where drug-drug interactions can cause
significant problems. This applies to some prescription drugs, over-the-counter
drugs, herbal and dietary supplement products, and recreational drugs. The use
of prescription drugs must be carefully monitored by the treating prescriber.
In addition, even modest amounts of alcohol can have significant effects on the
liver. St. John’s Wort, a popular herbal product for the treatment of
depression, is well known for causing some very significant changes in liver
metabolism.
The Process of Approving a New Drug by the FDA
There are four steps to getting a new drug approved by the FDA.
The pharmaceutical company has created a new chemical compound and done the preclinical
toxicology studies; then they have to submit an investigation of new drug
application (IND) to the FDA, followed by three phases of clinical trials. The FDA
will approve the final new drug application (NDA) based on the clinical trial
data. The FDA also monitors the post-market drug safety. (Drugs.com)
- Phase 1 focuses on safety. About 20 to 80
healthy volunteers are needed to establish a drug’s safety and profile,
and it takes about one year to complete. Safety, metabolism, and excretion
of the drug are also emphasized.
- Phase 2 focuses on effectiveness. Roughly 100 to
300 patient volunteers are required to assess the drug's effectiveness in
those with a specific condition or disease. This phase runs about two
years. Groups of similar patients may receive the actual drug compared to
a placebo or other active drug to determine if the drug has an effect.
Safety and side effects are reviewed.
- Phase 3 studies begin if evidence of
effectiveness is shown in Phase 2. Typically, several hundred to 3,000
patients are monitored in clinics and hospitals to carefully determine
effectiveness and identify further side effects. The manufacturer may look
at different doses as well as the experimental drug in combination with
other treatments. This phase runs about three years on average.
- Phase
4 studies gather additional information about a product's safety, efficacy,
or optimal use after approval. Post-marketing studies may take place in
groups of patients who are using the drug in a real-world setting. These
studies may identify additional uses, long-term effectiveness, and
previously undetected side effects. Rare side effects that occur in fewer
than 1 in 5,000 patients are unlikely to be seen in Phase 1 to 3 studies
before approval, but groups of patients this large are not usually studied.
These rare side effects are more likely to be found when large numbers of
patients use a drug after it has been approved and marketed.
Depression
Introduction
The World Health Organization (WHO) has launched the comprehensive
Mental Health Action Plan 2013-2030. Depression is a common mental illness; it
can affect anyone. Globally an estimated 3.8% of the population experience depression,
including 5% of adults (4% among men and 6% among women), and 5.7% of adults
older than 60 years. Approximately 280 million people in the world have
depression.(1) Depression is about 50% more common among women than among men. Worldwide, more
than 10% of pregnant women and women who have just given birth experience
depression.(2) More than 700,000 people die due to suicide every year. Suicide is the fourth
leading cause of death in 15-29 year-olds.
The twelve-month prevalence of major depressive
disorder in the United States is approximately 7%, with marked differences by
age group such that the prevalence in 18-29 year-old individuals is three times
higher than the prevalence among individuals age 60 years or older. Depression is the leading cause of
disability in the United States for individuals age 15-44 (DSM-5-TR), and ranks as the second most commonly seen
disorder in patients seeking treatment from primary care physicians. Data shows
the prevalence increased in the United States from 2005 to 2015 with steeper rates
of increase for youth compared with older groups. Non-hispanic whites showed a
significant increase. Morbidity and mortality associated with serious mood
disorders is enormous. Chronic and recurrent depressions significantly increase
risk for coronary heart disease, stroke, and osteoporosis (NIMH, 2003).
It is estimated that only 50% of those in the United States who
suffer from major depression seek treatment. Additionally, treatment received
is often very inadequate. In the United States most drug treatment for
depression takes place in primary care medical settings. Eighty-five percent of
prescriptions written in the United States for antidepressants are written by
physicians and nurse practitioners and physician assistants who do not have
specialty training in psychiatry. This is due in large part to managed care’s
efforts to cut costs by having the majority of psychiatric treatment take place
in primary care settings. In addition, there are shortages of psychiatrists.
Treatment outcomes in primary care medical settings may have poor response largely
due to the lack of adequate follow-up.
A Changing Treatment Landscape
The 1980s and 1990s saw the proliferation of HMOs and managed care
companies that attempted to streamline mental health care in order to achieve
cost containment. During this same time psychiatry training programs have
increasingly emphasized psychopharmacology as the mainstay of treatment for
clinical depression. Finally, pharmaceutical advances and marketplace forces
have had a substantial impact on shifting patterns of practice in the mental
health arena away from psychotherapy.
In many settings, psychotropics have become the primary or sole
form of treatment for many suffering from serious mood disorders. Yet, the vast
majority of patients treated either do not respond or only experience partial
responses.
Treatment Outcomes
How effective are antidepressants? All drugs must go through
rigorous trials prior to FDA approval, and must demonstrate clear superiority
over placebos. The currently available antidepressants have all passed the
test, but the outcome data is often spurious and plagued by methodological
flaws. First and foremost, in the majority of pre-FDA-approval efficacy studies,
patients selected for medication trials hardly resemble typical outpatient
populations. Patient selection excludes those who have had prior suicide
attempts, who have psychotic or bipolar symptoms, and who have comorbid medical
illnesses, or substance use or personality disorders. A paper by Zimmerman,
Posternak, and Chelminski (2002) looked at consecutive patients seen in an
outpatient setting who were diagnosed with unipolar/major depression. If
patient selection criteria as noted above were applied to this typical group
of depressed outpatients, 85% of them would be excluded from drug trials.
Complex comorbidity is the rule, not the exception, in usual clinical settings.
Thus, it may not be appropriate to generalize results from efficacy studies to
the real world of clinical practice.
Another significant flaw in the reporting of outcome data is the
common practice of excluding dropouts due to side effects from percentages of
responders (Gitlin, 2002). Thus, for most antidepressants studied, the positive
outcomes are inflated (see Table 1).
Table 1.
Antidepressant Treatment of Major Depression Outcome Results:
General Results Reported from Drug Efficacy Studies |
|
Commonly Reported Outcomes |
ITT Outcomes* |
Side-effects drop-outs |
15% |
15%** |
Responders |
60% |
50% |
Non-Responders |
40% |
35% |
*ITT: intention
to treat ** Drop-out rates due to side effects vary among new generation
antidepressants ranging from Wellbutrin, SR: 9%, to Paxil: 21%. |
A
large-scale clinical trial funded by the National Institutes of Health aims to
address whether antidepressants work or not. The Sequenced Treatment Alternatives to Relieve
Depression (STAR*D) study is the largest prospective clinical trial of major
depressive disorders ever conducted. It was a multicenter, nationwide
association of 14 university-based regional centers, which oversaw a total of
23 participating psychiatric clinics and 18 primary care clinics. Enrollment
began in 2000, with follow-up completed in 2004. All enrolled patients began on
a single selective serotonin reuptake inhibitor (SSRI) (citalopram) and were
managed by clinic physicians who followed an algorithm-guided acute phase treatment
through five visits over a 12-week course. Dosing was aggressive and focused on
maximizing the tolerable dose; if patients who were tolerating a medication had
not achieved remission (that is, complete recovery from the depressive episode)
by any of the critical decision points (weeks 4, 6, and 9), the algorithm
recommended increasing the dose. Patients whose depression did not remit after
this initial treatment were able to participate in a sequence of up to three
randomized clinical trials or levels. The results do show antidepressants work,
but with limitations, and it requires a combination of medications along with
cognitive therapy to achieve better clinical outcome. (Rush, et al., 2006)
What has become clear is that mood disorders are tremendously complex and heterogeneous disorders that involve numerous interacting variables, such as, genetic predisposition, underlying medical and neurobiological changes, life stressors, interpersonal relationships, and intrapsychic, cultural, and existential features. It is the appreciation of such complexity that has led to increased interest in integrative approaches to treatment (Preston, 2006).
Abnormal Neurobiology: A Compelling Case for the Use of
Antidepressants
A comprehensive review of the neurobiology of depression is beyond
the scope of this paper. However, let us consider a few important research
findings that have emerged during the past decade. Sixty percent of people
experiencing a major depressive episode exhibit a hyperactive
hypothalamic-pituitary-adrenal (HPA) neuroendocrine axis (see Figure 1). In
mammals, when danger or adversity is perceived in the environment, a number of
complex events take place in the nervous system, launching adaptive
fight-or-flight responses. One of the most important components of this
biological response is the activation of the HPA axis, which ultimately
releases glucocorticoid hormones into general circulation. In humans, the principal
glucocorticoid is cortisol. Cortisol operates to facilitate the release of
glucose into the blood stream and to increase blood pressure, both of which are
essential for mounting an effective fight-or-flight response.
Figure 1.
Hypothalamic-Pituitary-Adrenal Neuroendocrine Axis
See also, Le Doux (2015)

Hypo: Hypothalamus, CRF: corticotropin
releasing factor, PIT: pituitary gland, ACTH: Adrenocorticotropic hormone.
Cortisol enters circulation and is distributed throughout the
body. In the brain, the most prominent site for cortisol response is the limbic
brain structure, the hippocampus. In adaptive fight-or-flight responses,
cortisol activates the hippocampus to inhibit the HPA axis, operating like a
“brake”. Of course, if danger persists in the environment, the HPA axis is
continuously reactivated. However, when stressors subside, the
cortisol-mediated feedback loop shuts down the system, and thus plays a role in
affect regulation (LeDoux, 2017).
This hyperactivity of the HPA axis, seen in many cases of major
depression, results in a condition known as hypercortisolemia. Here,
cortisol levels are significantly elevated and have been shown to be
neurotoxic. These high, sustained, toxic levels of cortisol have a marked
impact on hippocampal nerve cells (e.g., causing dendrite retraction, cell death, and hippocampal atrophy). Hypercortisolism also may damage other neural tissue including the anterior cingulate, amygdala, and cerebellum, all of which have been
implicated in playing a role in affect-regulation). Over time, the result is
progressive brain damage that likely accounts for the deteriorating course seen
in many cases of untreated or poorly treated recurrent and chronic depression
and bipolar illness (Sapolsky, 1996). High cortisol levels in depressed
pregnant women cross the placental-blood barrier and may adversely affect the
fetus’s nervous system.
Additionally, depression reduces the gene expression of brain-derived
neurotropic factor (BDNF), which is a neuroprotective molecule known to
facilitate the repair of damaged nerve cells and to promote neuron regeneration,
that is neurogenesis, in the hippocampus. Molecular studies have also implicated
peripheral factors, including genetic variants in neurotrophic factors and
pro-inflammatory cytokines. Additionally, volumetric and functional magnetic
resonance imaging studies provide evidence for abnormalities in specific neural
systems supporting emotion processing, reward seeking, and emotion regulation
in adults with major depression (APA, 2022).
Antidepressant medications reduce cortisol levels (Heim &
Nemeroff, 2002; Nestler, Hyman, & Malenka, 2001) and reactivate the
production of BDNF, which can lead not only to clinical improvement, but also
to the birth of new nerve cells in the hippocampus (Kempermann
& Gage, 1999) (note: lithium, Depakote, Tegretol, Lamictal, Seroquel, and
Equatro, and regular exercise also increase the levels of BDNF). In this
way, antidepressants can play a crucial role in treating symptoms of depression
as well as protecting the brain from the effects of toxic levels of stress
hormones.
Diagnostic Issues
For many years, a distinction was made between so-called “reactive
depressions” or “psychological depressions” (by definition, depressions that
emerged in the wake of significant psychosocial stressors) and “endogenous
depressions,” presumably due to the effects of certain changes in neurobiology, such as,
genetically based mood disorders, secondary to various medical illnesses such as thyroid disease, or due to the impact of substances(e.g., alcohol or antihypertensive drugs). Currently, the distinction between endogenous and reactive depressions is less relevant. The critical diagnostic issues,as this relates to the decision to treat with antidepressants, include the following:
1. Marked dysfunction
(social, occupational, etc.) that lasts for more than two weeks or fails to
respond adequately to psychotherapy.
2. Depressed/sad mood and/or
loss of interest in life. Feeling hopeless, helpless, and/or worthless most days
or every day for at least two weeks.
3. Patients who are of
below-average intelligence, are significantly non-psychologically minded (i.e.,
unable to introspect), who refuse psychotherapy, where professional treatment
is not available, or who are too impaired to productively participate in
psychotherapy.
4. The presence of
neurovegetative symptoms (if these are sustained, i.e., present most days):
- Sleep disturbances (especially early morning awakening and
middle insomnia);
- Loss of libido;
- Anhedonia or a non-reactive mood;
- Appetite and weight changes (either increased or decreased);
- Marked fatigue;
- Psychomotor retardation or agitation; or
- Diurnal variations in mood and cognitive ability,
generally, more pronounced in the morning.
5. 66% of patients with Persistent
Depressive Disorder (previously called dysthymia) have been shown to be
responders to antidepressants and thus should also be considered for such
treatment.
6. Depression is caused
by a general medical condition or prescription medications. Typically, the
treatment strategy is to focus treatment on the general medical condition
directly and only use antidepressants if such treatment is unable to resolve
the depression. Likewise, a change in prescription medications (e.g., changing
the type of antihypertensive medication used to treat high blood pressure to a
different class of antihypertensive) may be effective in reducing depressive
symptoms. However, if not, the addition of an antidepressant may be considered.
7. Substance/Medication-induced
depression disorder. The essential character of substance/medication-induced
depression is directly due to physiological effects of a substance (e.g., a
drug of abuse, a medication, or a toxin exposure). We can do laboratory testing
(blood test and/or urine test) to detect a substance. The treatment goal is
absence from substance use or stopping the medication that can cause
depression. We can use an antidepressant to treat depression as well.
Psychopharmacology of Depression
Antidepressant Medications
Antidepressants and their daily adult dose ranges are listed below.
These medications have been developed since the late1980s and are considerably
safer and easier to tolerate than older-generation tricyclic and MAOI
antidepressants. The following table lists the commonly used antidepressants
from the different classes.
Classes of Antidepressants |
Generic Name |
Brand Name |
Typical Adult Daily Dose |
SSRIs (selective serotonin reuptake inhibitors) |
fluoxetine |
Prozac, Sarafem |
10-80 mg |
| |
paroxetine |
Paxil/Paxil CR |
20-50 mg |
| |
citalopram |
Celexa |
20-40 mg |
| |
escitalopram |
Lexapro |
10-20 mg |
| |
sertraline |
Zoloft |
50-200 mg |
| |
fluvoxamine |
Luvox |
50-300 mg |
SNRIs
(serotonin norepinephrine
reuptake inhibitors) |
venlafaxine |
Effexor/Effexor XR |
37.5-225 mg |
| |
duloxetine |
Cymbalta |
20-120 mg |
| |
desvenlafaxine |
Pristiq |
50-100 mg |
| |
levomilnacipran |
Fetzima |
20-120 mg |
TCAs (tricyclic antidepressants) |
amitriptyline |
Elavil |
25-300 mg |
| |
nortriptyline |
Pamelor |
25-150 mg |
| |
clomipramine |
Anafranil |
25-250 mg |
| |
sinequan |
Doxepin |
25-300 mg |
| |
impramine |
Tofranil |
25-100 mg |
MAOIs (monoamine oxidase inhibitors) |
phenelzine |
Nardil |
45-90 mg |
| |
selegiline |
Emsam (patch) |
6-12 mg/24h |
| |
tranylcypromine |
Parnate |
30-60 mg |
Atypical
antidepressants |
bupropion |
Wellbutrin (SR/XL) |
150-450 mg |
| |
mirtazapine |
Remeron |
15-45 mg |
| |
trazodone |
Desyrel |
100-300 mg |
| |
vilazodone |
Viibryd |
10-20 mg |
| |
vortioxetine |
Trintellix |
5-20 mg |
| |
dextromethorphan/bupropion |
Auvelity |
45/105 mg (two tabs daily) |
Pharmacologic Treatment Guidelines
During the past 20 years, large-scaled empirical studies have been
designed in order to establish guidelines for what is currently referred to as
“evidence-based medicine”. These include the STAR-D (Sequenced Treatment
Alternatives for Relieving Depression: a multi-site study sponsored by the
National Institute of Mental Health), the Texas Medication Algorithm Project,
and UCLA’s Targeted Treatment for Depression Program (Metzner, 2000). Treatment
guidelines in psychiatry have been influenced by three factors:
1. Marketplace variables,
such as pharmaceutical company advertising and decisions made by HMOs and managed
care companies to adopt cost-effective drug formalities).
2. Consensus, that is,
inviting “experts” to convene a panel and discuss pros and cons of various
medical treatments and agree upon best treatment strategies, and
3. Results
from a large amount of research regarding treatment outcomes. The latter of
these offer important information, but may be inherently flawed because most
psychopharmacology outcome studies are sponsored by drug companies who have a
vested interest in producing good outcomes. The major drawback here is that
many null studies never make it into the journals. Thus, it is difficult to
realistically evaluate the effectiveness of certain treatments with only
primarily positive outcome data available.
The large-scaled studies mentioned above are funded by the NIMH,
Texas Department of Mental Health, and a university, respectively, and thus may
more accurately reflect realistic outcome data, not influenced by the profit
motives of drug companies. Also, the studies are not ones of consensus
opinions, but rather based on empirical outcomes with very large groups of
subjects.
What is summarized below are the first of what promises to be a
growing body of evidence-based data that can suggest specific strategies for
the treatment of major depression.
First-Line Medication Choices: Major Depression
(Metzner, 2000; Shelton & Tomarken, 2001; Goodwin & Jamison,
2007).
Major depression as defined by DSM-5-TR criteria represents a
heterogeneous group of mood disorders that vary in terms of severity (mild to severe),
clinical/symptomatic presentation, and presumed etiology. A large body of
neuroscience research has strongly implicated that dysregulation of certain
central neurotransmitters may be associated with particular
psychiatric symptoms. Most individuals who experience a decreased availability
of neurotransmitters such as serotonin (5-HT), dopamine (DA), or norepinephrine
(NE) do not develop clinical depression (Delgado, Charney, Price, et al.,
1990). However, some do, which is likely due to underlying genetic or other
vulnerability factors. Among depressed subjects, inadequate functioning of
5-HT, DA, or NE can contribute to certain core depressive symptoms such as a
depressed mood, pessimistic and negative thinking, guilt, low self-esteem, and
fatigue. What has emerged during the past two decades of research are general
but consistent findings suggesting that beyond common core symptoms of
depression, particular neurotransmitter dysfunctions may be accompanied by, or
cause, specific symptoms:
- Serotonin
Dysregulation: irritability, impulsivity, suicidal impulses/behavior,
ruminations;
- Dopamine and/or
Norepinephrine Dysregulation: apathy, anhedonia, low motivation.
Closely paralleling these findings from neuroscience research are
data from empirical pharmacologic studies (e.g., Metzner, 2000), which have led
to the following guidelines in which particular symptomatic features point
toward first-line antidepressant medication choices.
Major Depressive Disorder (MDD) Treatment Algorithm: Choosing a
First-Line Antidepressant
In general, medications recommended for major depressive
disorder include Selective Serotonin Reuptake Inhibitors, which are used as the
first-line treatment (SSRIs: Prozac, Zoloft, Paxil, Luvox, Celexa, Lexapro). SNRIs
can be used as the second-line choice (serotonin and norepinephrine reuptake
inhibitors such as Effexor, Pristiq, Fetzima, and Cymbalta).
Antidepressants may have a side effect referred to as activation (affecting approximately 10% of patients), which is experienced as increased energy, anxiety, initial insomnia, and/or agitation, typically emerging several hours after taking the first dose (also this can occur shortly after dose escalations). In individuals with this acute onset, side effects often result in abrupt patient-initiated discontinuation (the side effects often scare patients and may make them very reluctant to go through another antidepressant trial).Thus although after 4-6 weeks of treatment SSRIs often begin to significantly reduce both depression and anxiety symptoms, the initial few weeks of treatment can be very challenging and many patients drop out of treatment. A popular and effective way to address this problem is to initially co-administer an anti-anxiolytic agent, such as abenzodiazepine (e.g. Ativan, a minor tranquilizer) to rapidly reduce anxiety, agitation, and drug-induced activation. In addition, since benzodiazepines begin to reduce anxiety within an hour of taking the medication, the experience for many patients is that they feel noticeably better, quickly. This is an added benefit because it often leads to hopefulness about treatment and enhances compliance. Generally, after one month of treatment the benzodiazepine can be phased out. This is often a very successful strategy, however there is one important warning. Since benzodiazepines can be drugs of abuse, the use of these agents is risky and not indicated if there is a history of alcohol or substance abuse. With an addiction history, some psychiatrists are now using the non-habit-forming antihistamine, hydroxyzine (Atarax; Vistaril), to target the activation, or low doses of trazodone (25-50 mg), or low-dose Remeron (7.5-15 mg) for initial insomnia. We can combine the dual-action antidepressants such as SSRIs with Wellbutrin or SNRIs with Wellbutrin to target different neurotransmitters, i.e., targeting 5-HT, DA and NE, and DA and 5-HT respectively. If the patient has only a partial response to an antidepressant, we can use an augmentation strategy to achieve a better result.
If a patient has major depressive disorder with psychotic
features, we can add an antipsychotic medication as an adjunctive treatment to
manage psychotic symptoms.
If a patient’s depression is very severe – the patient is unable
to eat, sleep, or function – the ECT treatment can result in up to 80% symptom reduction.
For patients who have atypical depression, symptoms
include: hypersomnia, appetite increase, carbohydrate craving with subsequent
weight gain, and severe fatigue. Other symptoms often associated with atypical
MDD are rejection sensitivity and anxiety attacks. Medications of choice are
SSRIs, monoamine oxidase inhibitors (MAOIs) and possibly Wellbutrin (APA,
2003).
It is important to exercise caution when treating atypical MDD
because many such individuals may be expressing the depressive phase of bipolar
illness and if treated with antidepressants alone there is a risk of switching
(i.e., provoking mania or hypomania) or cycle acceleration (the tendency to
cause increasingly severe or more frequent mood episodes). It has been found
that 80% of those suffering from atypical depression ultimately are discovered
to have bipolar disorder (usually, bipolar II). More will be said about
switching and cycle acceleration in the section below on bipolar illness.
Partial and Non-Responder Strategies
Clinically, 55% to 65% of patients treated with antidepressants
only experience a partial response or no response at all when receiving their
first antidepressant trial (Paykel, Ramana, Cooper, et al., 1995; Doraiswamy,
Kahan, Donahue, and Richard, 2001; NIMH: STAR-D, 2008). And as noted earlier,
those who do not reach full remission incur an increased risk of relapse
(Paykel, Ramana, Cooper, et al., 1995). STAR*D has shown that 50% of patients
will achieve remission if they stay in the treatment after two treatment steps (Rush et al., 2006). Empirical studies are beginning to provide databased treatment guidelines for partial or non-responders (see Texas Department of Mental Health, 2005 and NIMH: STAR-D Program, 2008).
It must first be recognized that a number of factors may account
for less than adequate antidepressant responses, including the following:
- Excessive use of
caffeine or energy drinks that can significantly interfere with slow-wave
(deep) sleep which can aggravate depression;
- Unreported substance
abuse (in particular, alcohol use/abuse which may both exacerbate depressive
symptoms and interfere with the metabolism of antidepressant medications);
- Unsuspected medical
illnesses (e.g., sleep apnea, restless legs syndrome, or thyroid disease);
- Failure to address key
psychological conflicts and/or family-based psychopathology;
- Choice of wrong class
of antidepressant medication (e.g., pharmacologically targeting the serotonin
system when in fact a particular patient’s depression is associated with
dysregulation of norepinephrine); and
- Chronic residual brain
impairment sustained during previous untreated or inadequately treated depressive
episodes (due to hypercortisolemia).
Partial Responder Strategies The highest yield next step
strategy is to progressively increase the medication dose (Trivedi & Klieber, 2001). This was also born
out in the STAR-D program that demonstrated better outcomes for very ill and
treatment-resistant cases by using high doses of antidepressants. Some patients
who are hyper-or rapid metabolizers require higher doses to achieve adequate
blood levels. The doses can be progressively increased if tolerated. If this
strategy is ineffective or impossible owing to emergent side effects, step two
is to augment (that is, to add an additional medication to the current drug).
Augmentation strategies often yield good responses in 35%-65% of those treated.
The following are common augmentation strategies that
often are successful:
- SSRI plus atypical
antipsychotic. Note: this strategy is often successful in treating partial
responders who have major depression with/without psychotic features. There
are four FDA-approved medications for augmenting antidepressants in the
treatment of major depression. They are Abilify, Rexulti, Seroquel XR, and
Vraylar;
- Antidepressant plus
lithium (generally lithium levels of 0.4-0.6 mEq/l);
- Antidepressant plus T3
(triiodothyronine), 25-50 micrograms per day;
- Combining Effexor and
Remeron (Stahl, 2000); and
- Antidepressant plus Transcranial
Magnetic Stimulation (TMS).
Non-Responder Strategies Once again, one must evaluate
issues involving adherence and possible substance abuse. Another consideration
is to make sure the diagnosis is major depressive disorder. Should these issues
be ruled out, then the highest yield next step strategy is to optimize the dose
(dosage increases, if tolerated). Should a high dose be reached and there is
still little or no response, then the next-step strategy is to change classes
of medications (e.g., switch from a serotonin-active drug [SSRI], such as Zoloft,
to a different class of antidepressants. (Trivedi & Klieber 2001).
Phases of Treatment for Major Depressive Disorder
There is general agreement among research groups that the
treatment of major depression involves three phases (American Psychiatric
Association, 2003; Texas Department of Mental Health, 2003):
Acute Phase:
Starts with the first dose and extends until the patient is asymptomatic. Since
symptoms have abated, many patients will naturally think that they no longer
need medications and will discontinue (against medical advice). At this point
in treatment, should patients discontinue, more than one-half will experience
an acute relapse within a few weeks (Stahl, 2013). Ongoing antidepressant
treatment, however, decreases the likelihood of acute relapse, necessitating
the next phase.
Continuation Phase: Continue treatment at the same dose for a
minimum of six months. If during this time period there are no breakthrough
depressive symptoms, then discontinuation can be considered. Gradual
discontinuation over a period of six weeks is strongly recommended to
avoid discontinuation withdrawal symptoms.
Maintenance Treatment: Lifelong treatment is strongly indicated for patients with
highly recurrent major depressions, those in their third or subsequent
episodes. Chronic treatment in an attempt to prevent recurrence can be helpful
in this regard as we manage other chronic illnesses such as hypertension and
diabetes. The maintenance of treatment is essential to prevent recurrence of depression.
Treatment for Other Types of Depression
Seasonal Depression: This often responds to high-intensity light therapy, generally
provided by the use of a commercially available light box or going out-of-doors
without sunglasses. The typical “dose” of light therapy is 10-30 minutes of
exposure to a light source that emits a minimum of 10,000 lux. The light has an
impact by striking the retina, which activates a specific nerve pathway (the
retinal-hypothalamic nerve). In most cases, high-intensity light therapy must
be accompanied by the use of antidepressant medications (Rosenthal, 2013). Note
that some seasonal mood disorders are associated with bipolar illness and thus
one must exercise caution in using bright light therapy to prevent a shift into
mania.
Premenstrual Dysphoric Disorder (PMDD): This disorder is characterized by repetitive cyclical,
physical, and behavioral symptoms occurring in the luteal phase of the normal
menstrual cycle. Symptoms may extend into the first few days of menses. Mood
symptoms are only seen for discrete periods of time prior to menstruation and
absent the rest of the month. The low-dose SSRI or SNRI is often helpful if the oral contraceptive or other alternative therapies, exercise or change of diet fail to control the symptoms. A higher dose may be prescribed if there is no response.
Postpartum Depression: Clinically, one in eight women
experience symptoms of postpartum depression, and the rate of depression
diagnoses at delivery is increasing. Technically, this can occur during
pregnancy but more commonly occurs after the birth of the infant. Especially if
there is a pre-existing depression, the onset can follow rapidly after
delivery. However, more commonly, the average onset is within four weeks
following delivery. Postpartum depression is distinct from what is often
referred to as “the baby blues”. Such a condition is not pathological but
rather reflects increased emotional sensitivity in general, for the full range
of emotions, including happiness as well as bouts of sadness or tearfulness.
It is thought that this phenomenon, common to most new mothers, occurs in 30%
to 80% of women following childbirth, with symptoms developing within two to three
days after delivery, peaking on the fifth day, and resolving within two weeks.
Postpartum depression, however, is quite different, with a prolonged
course and more severe symptoms. It often presents with symptoms of depressed mood,
anhedonia, weight changes, sleep disturbance, psychomotor problems, low energy,
excessive guilt, loss of confidence or self-esteem, poor concentration or
suicidal ideation. Many women tend to experience a severe episode and are
completely unable to hold or are disinterested in holding their babies. This
can profoundly interfere with critical early child-mother bonding (consequences
that may have a life-long impact). The greatest risk is postpartum psychosis.
Here, mothers do not interact at all with their children and in many cases,
they include the child in their delusional thinking. Estimates show that each
year in the United States 150 babies are killed by their mothers in the midst
of florid psychotic thinking and behavior. All women with postpartum psychosis
should be strongly considered for hospitalization.
The treatment is psychotherapy and/or medication. Antidepressants are recommended for more severe episodes.. Response rates are slow and such depressive and psychotic symptoms,if untreated, last longer than most unipolar depressive episodes. They may continue for one and a half or more years before spontaneous remission. Standard treatment requires at least two to four weeks to begin to cause a response. It is good that the FDA has approved two new medications (Zulresso and Zurzuvae) for treating postpartum depression.
Brexanolone (brand name Zulresso) is a drug approved by the FDA in
2019 with unique actions. It requires gradual intravenous infusion over a period of 60
hours and must be administered in the hospital. This is the first FDA-approved
drug for postpartum depression. It is presumed to have actions on
allopregnanolone, a neuro-steroid that has actions on the GABA system. With premenstrual depression and postpartum depressions, allopregnanolone
levels drop precipitously. Progesterone may be targeted by the drug but the precise
mechanism of action is unknown at the time of this course update. Typically,
30% of women experience a significant and rapid reduction in psychiatric
symptoms. The drug may have enduring positive effects for at least a month, but
longer-term follow-up studies have not been done to date. The cost of the
medication alone is currently $34,000, which does not include the cost of the
hospital stay and the clinicians involved. The most common side effects are
sedation, dizziness, dry mouth, loss of consciousness, and flushing.
Zuranolone (brand name Zurzuvae) is the first oral pill to treat postpartum
depression. It has been approved by the FDA since August, 2023. It can be given
with fat-containing food for 14 days. It works fast, in three days, and
remission is about 60% at six weeks. It improves anxiety symptoms as well. It has
a similar action as brexanolone. The common side effects are sedation,
dizziness, dry mouth, loss of consciousness, and flushing. However, the
medication trials haven’t included breastfeeding women.
New Treatments and Interventional Treatment for Major Depressive Disorder
While the most common treatment for major depression is to use a traditional treatment, that is, an antidepressant, , a better understanding of the pathophysiology underlying many mental illnesses has led to the recent increased use of treatments that require specialized administration and the creation of a subspecialty called interventional psychiatry. For example, we have mentioned Brexanolone (brand name Zulresso), an intravenousdrug for postpartum depression. We have esketamine, a nasal spray for refractory depression treatment as well. Other interventional treatments for depression are rTMS, vagus nerve stimulation, and ECT.
Esketamine (Brand name: Spravato) The drug ketamine has been used in
medicine and veterinary medicine for years as an anesthetic. It also has been
abused as a street drug because of its dissociative and hallucinogenic
properties (on the street it is often referred to as Special K). It
is also widely used in psychiatry mainly in ketamine clinics to treat severe,
treatment-refractory depression and especially acute, severe suicide risk. In
psychiatry, it is used off label and administered typically twice a week in the
clinic, because it requires IV dosing and a period of two to three hours of
patient observation afterward. This is because dissociative and some psychotic
symptoms exist for a period of several hours after dosing. Some severely affected
patients respond rapidly to the antidepressant effects. Also, there is often a
rapid decrease in suicidal ideation and impulses. It has also been used
successfully to treat patients with psychotic depressive episodes.
Recently, the active S isomer of Ketamine (esketamine; brand name Spravato) has been approved by the FDA (March, 2019), to treat refractory depression and/or acute suicidal risk. It is available in a nasal spray.
Because of its abuse potential, it is tightly regulated by both pharmacies and
treatment facilities. It must be administered in a treatment facility that has been
certificated in Spravato Risk Evaluation and Mitigation Strategy (REMS). The
duration of effects and even the frequency of dosing is largely unknown.
S-Ketamine is a glutamate NMDA antagonist, although its specific mechanism of
action is largely unknown. In many individuals, however, the effects are seen
within minutes to hours. The success of S-Ketamine opens the door for future
drug development because its action on glutamate is clearly different than
other, standard antidepressants.
Transcranial Magnetic Stimulation (TMS) In TMS, a series of magnetic pulses are administered via the scalp to stimulate neurons in areas of the brain associated with major depressive disorder (MDD). It is considered a non-invasive treatment for MDD. The initial recommended course of treatment is six weeks, but most improvement is seen in the first two to three weeks. After six weeks of treatment, many patients require ongoing maintenance treatment, which can be weekly or monthly based on response. The repetitive transcranial magnetic stimulation (rTMS) is the first one approved by the FDA, in 2008. As TMS technique has improved, we can do deep TMS, intermittent Theta burst stimulants, navigated TMS, and accelerated TMS to improve symptoms quickly.
Vagus Nerve Stimulation is a technique that was initially developed to treat severe epilepsy. It has been found to be effective in successfully treating about 50% to 60% of people who suffer from highly treatment-resistant depressions. The FDA approved it to treat chronic Treatment Resistant Depression (TRD) in 2005. A pacemaker-like device is implanted in the anterior chest wall (beneath the collar bone) and a wire extends up into the neck where it is wrapped around the left vagus nerve. Periodically, a mild electrical stimulation is delivered to the vagus nerve, which causes nerve activity that enters the brain. Due to the cost of the procedure and risks related to surgery, it has not been used widely in psychiatry.
Electroconvulsive therapy (ECT) Although ECT is used to treat psychiatric
illnesses ranging from mood disorders to psychotic disorders and catatonia, it
is mainly employed to treat people with severe treatment-resistant depression
(TRD). It is one of the most effective treatments for major depressive disorder,
and can significantly improve patients’ quality of life. During ECT treatment, the
patient can continue to be on antidepressants.
Herbs and Supplements for Major Depressive Disorder
A number of experimental treatments are widely used by people who
have experienced depression, especially people who try over-the-counter
supplements and herbs. To help symptoms of depression, people have tried omega-3
fatty acid supplementation (Peet and Horrobin, 2002), SAM-e, and St. John’s
Wort. It is recommended that if your patient is considering using them, the efficacy
and safety may not be established. It should only be taken with close
observation by their treating mental health professional.
Omega-3 Fatty Acids are dietary supplements that have some research support as
effective agents in reducing the severity of bipolar mood swings and major
depression (when added to standard medication treatments (Stoll, et al., 1999).
The responses in bipolar patients, however, have been disappointingly minimal,
while a number of studies do show positive responses when used in unipolar
depression. Doses that are used to treat or augment treatment of depression are
1000 to 2000 mg per day (EPA: the specific type of omega 3 that has an
impact on mood). Fish oil sources of omega 3 have greater bioavailability in
the brain and are more effective than omega 3 from flax seed or walnut oil. If
you want to know more about omega 3, please see NIH omega-3 fatty acids: health
professional fact sheet.
SAM-e (S-adenosylmethionine) is a naturally occurring bio-molecule found in most living
cells. It is felt to be necessary for carrying out a number of important
intracellular chemical reactions. SAM-e has been used in Europe for more than
20 years as a treatment for depression. Some studies have shown it to be
equally effective when compared to prescription antidepressants, while others do
not show any benefits or only minimal effectiveness. Most notable is the
virtual lack of side effects. Doses for the treatment of depression range from
400-1600 mg per day, although recent investigations indicate that often the
higher doses (1200-1600 mg per day) may be necessary for effectively reducing
depressive symptoms. SAM-e may be useful in treating bipolar depression,
however, one must exercise caution because it has, in fact, been found to
switch people with bipolar depression into states of mania.
St. John’s Wort is an over-the-counter dietary supplement that has been found to
have antidepressant properties. A meta-analysis of 22 studies has shown that
St. John’s Wort is equally effective to prescription antidepressants in the
treatment of mild-to-moderate depression (Lined, et al., 2008). This herbal
remedy is generally well tolerated with few if any side effects. There are
reported cases of possible infertility problems associated with its use,
although it is as yet unclear whether this is a common side effect. St. John’s
Wort requires daily dosing of 900-1800 mg. (taken in three divided doses), and
typically the first signs of symptom improvement take about six weeks to
emerge. Thus, the onset of action is somewhat longer than that seen with
prescription antidepressants. And as with any other treatment that has
antidepressant properties, St. John’s Wort can potentially provoke mania in
people with bipolar illness. Caution: although St. John’s Wort, when it is the
only medication being taken, appears to be quite safe, it has been found to
cause some very significant drug-drug interactions. It is strongly advised that
patients never take St. John’s Wort without first consulting with their
physician or pharmacist.
Oxytocin is
a hormone normally produced in the hypothalamus and released by the posterior
pituitary. Oxytocin may
influence depressive disorders by modifying stressor reactions. Oxytocin interacts
with the HPA axis, monoamines, and inflammatory factors. Social sensitivity
might be modulated by oxytocin thereby affecting negative affect (Mcquaid,
2014).
Combined Psychotherapy and Pharmacotherapy
Aside from its role as a primary treatment for some types of
depression, a number of studies have demonstrated that psychotherapy can
enhance treatment outcomes when combined with drug treatment (McCollough,
2000) and may contribute significantly to aiding in relapse prevention
(Reynolds, Frank, Perel, et al., 1999; Evans, Hollan, DeRubelis, et al., 1992;
Thase, Greenhouse, Frank, et al., 1997). Several authors including MacFarlane
(2003) and Whisman & Uebelacker (1999) advocate treatment models that
combine couple therapy with individual and pharmacological interventions for a
more integrated treatment approach. Additionally, the psychotherapist is in the
best position for closely monitoring adherence, side-effect problems, and
clinical response to medication treatment. This is especially important if the
client is being treated in a primary care setting where the therapist can
collaborate with the physician in order to optimize treatment outcomes.
Antidepressants
and Increased Risk for Suicide |
There has been a good deal of media
attention regarding potential risks of antidepressants and increased
suicidality (especially in children and adolescents). The initial concern
came from studies in England that raised concerns about increased suicidality
in young patients treated with the antidepressant Paxil. In this study, which
included 1,300 patients, Paxil was compared to placebo and reports of
increased suicidality were seen in 1.2% of placebo and 3.4% of Paxil-treated
subjects. This difference is statistically significant. It is important to
note that there were no actual suicides in this group of youngsters. A
problem is that “suicidality” has been very loosely defined in this and other
studies. Most times it includes reports of increased thoughts about suicide,
suicide gestures, non-lethal-intent, tension-reducing self-harm (as is often
seen in borderline personality disorders), and in one instance a report of a
child slapping herself. (Brown University, 2004). Of course, actual suicides
and lethal attempts are also included under this umbrella of suicidality. The
FDA has responded to concerns about increased suicidality by requiring drug
companies to issue warnings about the use of these drugs with younger
clients. Since the media blitz regarding antidepressants and suicidality in
youngsters, prescriptions written for children and teenagers have decreased
by 30%-40%. There has been a corresponding increase in reported suicidal
ideations among depressed youngsters, but not an increase in
actual suicides. The FDA has reviewed a number of studies focusing on the use
of antidepressants in youth. The total number of subjects in these studies is
4,400. The risk of treatment-emergent suicidal events is approximately 2% in
those treated with either no medications or placebo and 4% of those treated
with antidepressants. The most common “suicidal event” is suicidal ideations.
In the group of 4,400 subjects, there were no actual suicides.
What is clear is that untreated major
depression carries extreme risks of potential suicide, antidepressants take
several weeks of treatment before the first signs of clinical improvement, and
depression can worsen during this startup period of treatment. This can
happen in both drug treatment as well as psychotherapy, and thus therapists
must be watchful for treatment-emergent suicidality regardless of treatment
used. Antidepressants can cause an acute increase in anxiety and agitation
during the first ten days of treatment (i.e., activation: affecting about 10%
of adults treated and 10%-15% of children and young adolescents treated)
which could contribute to increased dysphoria. Maybe more importantly, among
teenagers presenting with major depression, 40% turn out to have bipolar
disorder. This is true for 50% of pre-adolescent children with major
depression. Antidepressants are known to pose risks for precipitating mania
in bipolar patients. In younger people, mixed mania is very common in bipolar
disorder and dysphoric mania is accompanied by significant suicide risk,
Thus, in treating major depression it is very important to make sure the
depression is not associated with bipolar disorder before prescribing
antidepressants. Finally, it is always important to differentiate between
media hype and scientific data.
In
2004, the FDA required a black box warning related to increased
risk of suicidality in pediatric patients for all antidepressant drugs, and
in 2007 extended the warning to include patients up to age 24. |
|
Bipolar Spectrum Disorders
Introduction and Diagnostic Issues
Bipolar disorder is a common type of mood disorder that is a
recurrent and often chronic mental illness marked by episodes of hypomania or
mania and depression, associated with a change or impairment in function. It
includes bipolar I disorder, bipolar II disorder, cyclothymic disorder, substance/medication
induced bipolar and related disorder, bipolar and related disorder due to
another medical condition and other specified bipolar disorder, and unspecified
bipolar disorder. The lifetime prevalence of bipolar disorder type I in the US
is estimated to be between 0.5% and 1.0%, affecting men and women equally. The
lifetime prevalence of bipolar II disorder in the US is estimated to be between
0.5% and 1.1%, with women more likely than men to be affected.
This is an introductory course on bipolar spectrum disorder. If you want to learn more, please see the course on this website called Bipolar Spectrum Disorders: Diagnosis and Pharmacologic
Treatment.
Symptoms of Classic Mania
- Euphoria or an
inflated sense of self-worth
- Restlessness,
agitation, hyperactivity
- Decreased need for
sleep (e.g., sleeping 3-4 hours per night, yet without daytime fatigue)
- Racing thoughts and
rapid, pressured speech
- Poor judgment and
impulsive behavior, e.g., spending atypically large amounts of money, driving
fast/recklessly, marked alcohol or drug abuse, promiscuity and engaging in
unsafe sex
- Psychotic symptoms can
occur
Symptoms of Hypomania
Hypomania is a milder version of mania that typically involves
much less intense mood symptoms. The duration of hypomania is at least four
days and is frequently not noticed as being a sign of illness by the person
experiencing it (although most times family members are more clearly aware of
the mood changes and increased energy). During the hypomanic episode, the
person can feel highly motivated and productive, is witty, gregarious and
“upbeat”, although there is often underlying irritability. One very common
sign of hypomania is a decreased need for sleep with no daytime fatigue.
Symptoms of Mania or Hypomania with Mixed Features
- Dysphoria or negative or pessimistic
mood
- Diminished interest or
pleasure
- Fatigue or loss of energy
- Feelings of worthlessness
or excessive guilt
There are five main subtypes of bipolar disorder:
- Bipolar I: severe manic (classic or dysphoric) and
depressive episodes (often with periods of normal/euthymic mood between
episodes).
- Bipolar
II (including hypomania and depression): characterized by frequent, severe and prolonged
depressions with periodic, brief episodes of hypomania. A normal/euthymic mood
can occur between episodes, but often during these in-between times there is a
low-grade/mildly depressed and/or irritable mood. Judd, et al. (2002) studied
the course of bipolar II disorder for a period of ten years and found that 15%
of days were spent in major depressions, 40% in minor depressions and only 1.4%
in hypomania.
- Cyclothymia: a chronic, fluctuating mood disturbance in
which symptoms of mood changes do not meet the full criteria for depression and
hypomania. (Note: this less severe version of bipolar can become worse with time
and up to one-half of people with cyclothymia eventually convert to Bipolar I
or Bipolar II.
- Substance/Medication-Induced Bipolar and Related Disorders: By the history taken from the patient, their physical examination, or laboratory findings, the symptoms of mania or hypomania are found to have developed during or soon after substance intoxication or withdrawal, or after exposure to or withdrawal from a medication. We know substance use and medication can induce mood changes.
- Bipolar and Related Disorder Due to Another Medical Condition: It is clear from the history taken from the patient and the physical examination, that the mood changes (mania, hypomania, and depression) are directly caused by the medical condition.
If the patient’s symptoms do not fully meet the criteria for
mania, hypomania, or depression, however, and their symptoms still cause
clinically significant distress or impairment in social, occupational, or other
important areas of functioning, we call them “Other Specified Bipolar and
Related Disorder and Unspecified Bipolar and Related Disorder”.
“Rapid cycling” is one of the specifiers we commonly hear about.
It requires at least four mood episodes in 12 months and the mood episodes have
met the criteria for manic, hypomanic, or major depressive episodes. These
episodes can occur in any combination and order.
Untreated or poorly treated bipolar illness leads to disaster.
Careers and marriages can be ruined, physical health problems abound, and there
is a high rate of suicide (19%-20% lifetime risk). If not treated, most cases
of bipolar disorder become progressively worse, likely owing to kindling
effects. The sooner this illness can be diagnosed and properly treated, the
better.
Treatments for Bipolar Disorder
Although the focus of this course is on psychopharmacology, we
will also briefly address adjunctive treatments. Medication treatment alone is
never adequate to fully control bipolar disorders. Treatment must have a
two-pronged focus: bringing to an end the current manic or depressive episode,
and relapse prevention. With proper medical treatment, most people can
experience a marked decrease in episode frequency and severity.
Lifestyle Management
People with bipolar illness have very unstable and fragile neurobiological
mechanisms for affect-regulation. Extreme emotional lability and mood
episodes can be triggered by a number of environmental, psychological, and
physiological stressors. Before a discussion of medication treatment, we will
address lifestyle management issues. It is especially important to regulate
one's lifestyle closely, because without this, medical treatments often are only
partially effective (Malkoff-Schwartz, et al., 1998). Most important are:
- Maintain regular bedtimes
and awakening times. Such regularities in sleep patterns are crucial;
- Avoid substance abuse
and alcohol use/abuse like the plague (substance abuse is very common in
bipolar disorder and often significantly aggravates the illness);
- Avoid sleep
deprivation, shift work, and crossing time zones;
- Avoid or greatly
minimize caffeine use since it can significantly disrupt the quality of sleep;
and
- Keep the amount of
bright light exposure (e.g., sunlight) and the amount of physical exercise
consistent each day year-round.
Medication Treatments: General Considerations
General references:
- Hirschfeld, et al.
(2002): Bipolar disorder Practice Guidelines
- Goodwin and Jamison
Manic Depressive Illness (2007)
- Texas Department of
Mental Health (1998) Texas Medication Algorithm Project
- National Institute of
Mental Health (2006) STEP-BD Program
- American Psychiatric
Association: (2002) Bipolar Disorder Practice Guidelines: Second Edition.
Currently, there are many medications that are approved by the FDA
for the treatment of bipolar disorder: Lithium, Depakote, Equetro, Lamictal,
Symbyax, Zyprexa, Risperdal, Seroquel, Geodon, Abilify, Latuda, Saphris,
Vraylar, and Caplyta. However, a number of other effective drugs are in common
use. The use of medications not approved by the FDA for the treatment of
certain conditions is referred to as “off-label use.” It is important to note
that off-label use of medications is a very common practice in every branch of
medicine. Commonly used as off-label medications for bipolar disorders are antiseizure
medications such as Tegretol, and antipsychotic medications such as Thorazine
and Haldol.
Bipolar spectrum disorder management also requires a long-term
management plan that includes medication treatments, continuation of medical
follow-up, and adjunctive psychosocial therapies such as a healthy lifestyle
including sleep hygiene, exercise, and stress management.
In addition, the medication choice always must take into
consideration the ultimate goal of preventing recurrences, a lot of which ultimately
depends on long-term medication tolerability.
Recent surveys reveal that in the United States only 11% of people
being successfully treated for bipolar disorder are taking just a single drug (i.e.,
mono-therapy); thus, this is the exception and not the rule. On average, most
people being treated are taking two or four medications simultaneously. The
reason for this is simple: medication combinations are often clearly superior
to mono-therapy for most people suffering from bipolar disorder.
All medications have side effects and unfortunately, the drugs
used to treat bipolar disorder are known to produce significant side effects
for the majority of people being treated. Side effects, at times, are mild and
easy to tolerate. But often they are more noticeable and in rare instances they
can be dangerous. In every single case, once the current mood episode has
subsided, people with bipolar disorder must continue to take certain
medications (mood stabilizers) to help prevent or reduce the likelihood of
recurrence. This is absolutely essential! However, some estimates suggest that
as many as 90% of people who start medical treatment for bipolar disorder will
recover from their first episode, but within weeks or several months, will stop
taking the medications (against medical advice). The most common reasons for
doing so are understandable:
1. patients are plagued by unpleasant side
effects;
2. there is negative stigma regarding mental illness; and/or
3.
they conclude that the episode they experienced is not really bipolar disorder
but just a single episode and that there will not be recurrences. This
conclusion is borne of hopefulness that this is not really going to be a
recurring illness (Pope and Scott, 2003). These reasons for discontinuing the
medication are entirely understandable, but they almost invariably lead to the
emergence of another episode (this may occur within a few months following the
initial episode, but more commonly occurs several years later).
Many side effects can be managed by dosage adjustments or by
switching to other medications. This is one reason that, most times, people
will need to go through systematic trials on a variety of medications to
determine which ones are the most effective and also which drugs are best
tolerated for any given individual. Every effort should be made to find the
right medication or medication combinations in an attempt to minimize side
effects. And this is often something that can be accomplished. However, it is
often the case that it takes a year of trials on various medications to finally
discover the specific medication or medication combinations that will be
effective and that will be best tolerated. This is the rule and not the
exception … it is very important for patients to not feel too discouraged if the
first medications used are less than optimally effective or if they have problematic
side effects. A sign of a competent and compassionate psychiatrist is his or
her willingness to be persistent in carrying out systematic medication trials
until the best treatment is finally identified. Sometimes, side effects can be
minimized, however, many people end up having to find ways to tolerate some
side effects. Obviously, this is not pleasant, but it is ultimately necessary
to reduce or eliminate severe mood swings. And unfortunately, a very small
number of people are simply unable to tolerate any bipolar medications.
Medication Treatments: What are Realistic Outcomes?
Bipolar disorder is like a number of other chronic medical
conditions, such as emphysema, asthma, or arthritis. It is not a condition
that can be cured by currently available medications. It is a recurrent
disease. However, the medications discussed below can be effective in
relieving many of the more serious symptoms of bipolar illness and often can
reduce the frequency of mood episodes for most people, if patients receive appropriate
treatment and stick with it. Good news and not so good news: with aggressive,
appropriate, and ongoing medication treatment, and if the treatment is started
during the first or second mood episode, about 25% of people will not
experience major recurrences. That is, in about one out of four people, the
medications are successful in preventing relapses (please note: if the first
appropriate medical treatments begin after the second episode, typically
treatment becomes somewhat more challenging and the outcomes are not quite as
robust). For the majority of other people receiving treatment after
the first episode, if they stay on medications, the recurrence rates for
severe episodes can be reduced by about 75% and hospitalizations can often be
avoided. Subsequent episodes that do occur tend to be mild depressions and
hypomanias (Gitlin, 2002). The majority of people being
treated for bipolar disorders, however, are frequently non-adherent with medical
treatment and recurrent episodes are the rule, not the exception. One of the
keys to long-term treatment success rests on medication tolerability. This is a significant challenge since most bipolar medications carry high
risks of unpleasant side effects.
For many patients, taking medications when they feel well is
counter-intuitive. However, the picture is clear that bipolar disorder is
always recurring, and over a period of time there is a tendency for episodes to
become increasingly severe and harder to treat. Medication treatments are far
from perfect, but they have the kind of effectiveness that can substantially
reduce suffering, keep families together, avoid catastrophes, and save lives.
Bipolar Medications
There are three major classes of psychiatric medications used to treat bipolar disorder. These include Lithium, Anticonvulsants, and Antipsychotics. Other medications have been found to be effective in treating various symptoms of bipolar disorder. Generic and brand names (registered trademarks) are listed below.
Lithium
Generic Name |
Brand Name |
Typical Adult Daily Dose |
lithium |
Lithonate, Eskalith |
600-1800 mg |
Therapeutic blood levels
| Acute mania: |
0.8-1.2
mEq/l |
Prophylaxis: |
0.6-0.8 mEq/l |
Anticonvulsants, mood stabilizers
Generic Name |
Brand Name |
Typical Adult Daily Dose |
Divalproex sodium |
Depakote |
750-1500 mg |
carbamazepine |
Tegretol, Equetro |
800-1600 mg |
Oxcarbazepine (off-label) |
Trileptal |
600mg-1200mg |
lamotrigine |
Lamictal |
50-200 mg |
Therapeutic blood levels
Depakote
blood levels |
50-125
mcg/ml |
Tegretol
blood levels |
4-12
mcg/ml |
Trileptal
blood levels |
not
yet established |
Lamictal
blood levels |
not
necessary to monitor |
Atypical Antipsychotics, also referred to as second
generation antipsychotics
Generic Name |
Brand Name |
Typical Adult Daily Dose |
olanzapine |
Zyprexa |
5-20 mg |
risperidone |
Risperdal |
2-6 mg |
quetiapine |
Seroquel |
200-800 mg for bipolar I disorder, manic
and 300 mg for bipolar depression |
ziprasidone |
Geodon |
40-160 mg with food |
aripiprazole |
Abilify |
15-30 mg |
asenapine |
Saphris |
5-20
mg |
lurasidone |
Latuda |
20-120
mg with food |
cariprazine |
Vraylar |
3-6
mg |
Lumateperone |
Caplyta |
21
mg-42 mg |
Other Medications We Can Use to Treat Bipolar Spectrum Disorder
Antidepressant/Antipsychotic combinations
Note: Symbyax,
a combination of Prozac and Zyprexa, was approved by the FDA in 2004 for the
treatment of bipolar depression. It comes in the following formulations:
(Zyprexa dose/Prozac dose): 3 mg/25 mg, 6/25 mg, 6/50 mg, 12/25 mg, 12/50 mg.
Benzodiazepines, also called anxiolytic medications or minor
tranquilizers
Benzodiazepines: Generic and Brand Names and Typical Adult Daily
Doses:
Generic Name |
Brand Name |
Typical Adult Daily Dose |
diazepam |
Valium |
5-10 mg |
clonazepam |
Klonopin |
0.5-4.0 mg |
lorazepam |
Ativan |
2-10 mg |
Note: Alproazolam (Xanax) is the one benzodiazepine that can
aggravate mania.
Targets for Medication Treatment
There are three primary goals in medication treatment for bipolar spectrum
disorder: dealing with potentially dangerous emergency issues (e.g., extremely
severe agitation or suicidal impulses), resolving the current episode (whether
mania or depression), and relapse prevention. The choice of medications used
always will be influenced by these goals. In addition, obviously, the medication
choice will also be dictated by the need to minimize side effects.
Getting Started with Medication Treatment: Emergency Medication
Treatments and Laboratory Tests
Sometimes there is a need for emergency treatment; for example, if
a person is experiencing a sudden onset of severe manic agitation (which may
include extreme restlessness, agitation, impulsivity, severely impaired
judgment and/or aggression), and/or serious suicidal impulses during a
depression. At such times, acute medical treatment may be necessary.
When there is such a crisis, hospitalization is almost always
necessary. Emergency medical treatments for agitation include the use of either
benzodiazepines (anxiolytics such as Ativan, Klonopin, or Valium) or antipsychotic
medications (such as Zyprexa, Risperdal, or Haldol). These two classes of drugs
are often very effective in rapidly reducing agitation. On occasion, there is a
need for emergency medical treatment for very severe depression (where there is
either a grave suicide risk or refusal to eat accompanied by severe weight
loss). In such cases, ECT (electroconvulsive therapy; “shock” treatments) can
be successfully used. ECT is also very effective for the emergency treatment of
severe mania.
If the situation is not extremely urgent, then it is commonplace
to order some pretreatment laboratory tests. This is done for two purposes. The
first is to rule out the possibility that the mood symptoms may be caused by a
primary medical illness (such as thyroid disease) or substance abuse (e.g., to
see if manic symptoms are being caused by methamphetamine use). The other
reason has to do with the tendency for many of the bipolar medications to cause
significant changes in a variety of bodily functions. Mood stabilizers in
particular are known to affect a broad range of organs and glands, especially
when they are taken for prolonged periods of time. Thus typically, pretreatment
labs include measures of cardiac, kidney, liver, and thyroid functioning as
well as a complete blood count. Laboratory monitoring of blood levels of
certain medications may also be required. This is routinely done for the
following mood-stabilizing medications: lithium, Tegretol, Trileptal, and
Depakote.
Treatment Guidelines: Mania
Several classes of psychiatric medications have been found to be
effective in treating acute manic episodes:
- Anticonvulsants:
Depakote, Tegretol, Trileptal
- Antipsychotic medications:
Second generation antipsychotics (SGAs: see chart above) and Haldol (this final
drug is an older generation antipsychotic that is still sometimes used)
- Benzodiazepines (anxiolytics
and minor tranquilizers)
These medications generally require seven to ten days of treatment
before you see an onset of action and symptom-reduction, except for benzodiazepines,
which can calm and reduce agitation quickly. Once symptoms begin to be reduced,
continued treatment for several weeks will often be necessary to eliminate
acute manic symptoms. As noted earlier, 90% of people will ultimately be
treated with two or more medications simultaneously to achieve the best
outcomes.
There are three stages in the medical treatment of mania:
- Reduce extreme
agitation (the goal is to get agitation under control within a few hours).
Severe agitation can be dangerous to the patient as well as to others around
them and this must be addressed as soon as possible;
- Reduce core manic
symptoms such as restlessness, sleeplessness, rapid speech, paranoid ideas, etc.;
and
- Begin treatment for
relapse prevention.
Stage One
As mentioned above, patients are usually treated in the hospital, and
antipsychotic medications and benzodiazepines are the best medications for
treating acute agitation; they quickly produce substantial sedation, calming,
or sleep. It is important to note that although antipsychotic medications do
successfully treat psychotic symptoms (such as hallucinations), they also are
effective anti-manic agents. Most mood stabilizers require the seven to ten day
period of time before symptom reduction, but with one notable exception: the
anticonvulsant mood stabilizer Depakote, which when given in loading doses, can
begin to show anti-manic effects in about four days. Often, once severe
agitation has subsided, benzodiazepines are gradually reduced and then within a
few days discontinued. This may also be true for antipsychotic medications.
However, often antipsychotic drugs may continue to be used for a prolonged
period of time.
During stage one of treatment, as mentioned above, a number of lab
tests are often done to monitor the early effects of the drugs.
Stage Two
The choice of medications used to treat core symptoms of mania is
important and often complex. As noted above, there are different symptom
presentations of mania which can be classic mania or mania with mixed features.
A considerable amount of research has been done to discover which medications
are best suited for treating particular patients. Dozens of large-scale
research studies have been conducted in recent years and specific treatment
guidelines have been developed that are very useful in helping physicians decide
on initial medication choices (see below). However, the fact is that each
person will have a number of factors unique to them that will influence
the choice of medications, such as age, gender, body weight, history of
allergies to medications, liver metabolism rate, the presence or absence of
other medical conditions, and other medicines being used to treat such
conditions. Your patients must anticipate that it is extremely common for
psychiatrists to make initial medication choices, begin treatment, and then
during the following weeks or months make what are often frequent changes in
the doses or medications prescribed.
“Classic Mania” (with euphoria, expansiveness, upbeat mood,
irritability, etc.) has been found to respond best to treatment with lithium or
Depakote. Other anticonvulsant mood stabilizers or atypical antipsychotic
medications often can treat classic mania, but in head-to-head comparisons,
lithium and Depakote appear to be the best first-line medications for this type
of mania.
With Mania with Mixed Features (agitation, decreased need for
sleep, extreme irritability, insomnia, feelings of despair, hopelessness, etc.),
there is some controversy regarding the treatment of dysphoric mania. Currently,
there are no FDA-approved medications to treat mixed features. However,
clinically we use different classes of mood stabilizers to manage symptoms.
The treatment of childhood-onset bipolar disorder is beyond the
scope of this course; however, a few brief comments will be made. When mania
occurs in pre-pubertal children, it almost always presents as a form of
dysphoric mania with rapid cycling and marked irritability. With adults, the
general strategy is to begin treatment with one mood stabilizer and only later
add additional medicines if they are needed (although as noted above, sometimes
two drugs are started very early in treatment). Preliminary research has rather
strongly indicated that most children suffering from mania ultimately end up
taking two or more mood stabilizers (this is required for most to effectively
eliminate manic symptoms). Thus, there currently is a trend to begin treatment
with children using two mood stabilizers (often this combination is Depakote
and lithium). It is generally felt that the much higher success rate with two
mood stabilizers outweighs the added side effects of using two drugs. Also, it
is felt that the earlier you can put a lid on mania and arrest its development,
the better … to do so matters not only regarding the current episode but may also
have a positive effect on reducing the severity of future episodes.
Treatment-Resistant Mania
For people who experience very severe mania that does not respond
to more traditional treatments, there are a number of options.
The antipsychotic medication clozapine (brand name Clozaril) has
been found to be effective in some cases of treatment-resistant mania. This
drug has antipsychotic effects (e.g., for treating hallucinations or delusions). It is proving to be effective not only for treating mania but also for relapse
prevention. Unfortunately, Clozaril has more significant side effects, some of
which are potentially dangerous, such as agranulocytosis, neutropenia,
myocarditis, and cardiomyopathy. ECT (electroconvulsive therapy) is a safe and
highly effective treatment for severe mania.
Stage Three: Maintenance Treatment
Once the current manic episode is completely controlled, it is
common practice to continue medications, even though there are no obvious
symptoms. This is necessary because it is clear that once symptoms subside, if
one discontinues, then the acute relapse rate can be as high as 85%. Thus, for
a period of several months, typically medication treatment is continued and
often at the same doses used during treatment of the acute phase of the
episode. This phase of treatment appropriately is called maintenance treatment.
In general, the medications used to treat mania are considered to
be very effective for most people experiencing a manic episode. Bipolar
disorder is a lifelong illness and all experts agree that a lifelong treatment
is required. It is important to know that most medications used to treat
bipolar disorder do have side effects that may emerge with very long-term use,
thus necessitating periodic lab tests to monitor blood and various glands and
organ system functioning. What is clear is that failure to treat (or to
adequately treat) bipolar disorder almost always leads to disaster.
The medications for which the best data exist for long-term
maintenance treatment are the following mood-stabilizers: lithium (the best
data), Lamictal, and Depakote. Most people on maintenance treatment will
continue to take several medications. However, longer-term treatments generally
do not include treatment with benzodiazepines or antidepressants. These
medications may destabilize bipolar patients. In the end, what matters is
the ability to find medication combinations that achieve long-term
tolerability.
Treatment Guidelines: Bipolar Depression
Several classes of psychiatric medications are often used to treat
bipolar depression:
- Lithium (to treat
bipolar depression, lithium levels generally have to reach 0.8 or higher);
- Symbyax (a combination
of Zyprexa and Prozac. FDA-approved to treat bipolar I and bipolar II
depression);
- Lamictal (note:
among bipolar medications, Lamictal has the most favorable side-effect profile,
even though the FDA has not approved it to treat bipolar depression); and
- Antipsychotic
medications: Seroquel/Seroquel XR, Latuda, Vraylar, and Caplyta have been
approved by the FDA to treat bipolar I depression. Caplyta is the only
medication that can be used to treat bipolar I/ II depression as monotherapy
and/or adjunctive therapy with Lithium and Depakote.
It is important to underscore that many treatments that ordinarily are effective in reducing unipolar depression, such as, antidepressants, carry a
risk of provoking manic episodes (a phenomenon referred to as “switching”) or
causing cycle acceleration (this refers to a gradual, over-all worsening of
bipolar disorder in which, over time, there is an increased frequency of
episodes and episodes tend to become more severe and more difficult to treat).
Switching and cycle acceleration have been documented with the use of
antidepressants and thus these drugs should only rarely be used in the
treatment of bipolar depression (Ghoemi, et al., 2001; Post, et al., 2001).
Some recent studies suggest that the addition of an antidepressant to a mood
stabilizer may be beneficial in treating bipolar depression, however, there is
not clear evidence to support this use. Excessive bright light exposure (which
can treat some types of seasonal depression) has also been associated with provoking
manias. Additionally, three popular over-the-counter products that have
antidepressant properties may, likewise, cause switching or cycle acceleration:
St. John’s Wort, 5-HTP, and SAM-e.
There are also three stages in the treatment of bipolar
depression.
Stage One
In the event of life-threatening symptoms such as strong suicidal
impulses or refusal to eat, ECT is a highly effective treatment. The treatment
approach is much the same as that used to treat acute mania. The other emergency
treatment is hospitalization. Unfortunately, aside from ECT, most approaches to
treating depression require several weeks before one is likely to see
symptomatic improvement.
Stage Two
Start with a medication with proven efficacy in treating bipolar
depression. The drugs that have the best track record of effectiveness, as
noted above, are Seroquel/Seroquel XR, Symbyax, Latuda, Vraylar, and Caplyta. We
also use Lamictal to treat bipolar depression. Lamictal has not been approved
for this use, clinically, however, it is often effective in treating
bipolar depression with good tolerability from patients. There is an important
consideration when using Lamictal: as treatment is begun, it is required that
Lamictal be given in small doses and that dosage changes are done very
gradually during the first four to six weeks of treatment. This is done to
avoid inducing a potentially dangerous rash called Stevens-Johnson syndrome that
can occur if there is rapid dose escalation. Fortunately, since the use of
gradual dosing has been adopted, the risk of Stevens-Johnson is almost nil. Lithium
has been shown to have antidepressant effects when administered as a
monotherapy in bipolar depression (although not in unipolar depression).
Stage Three
This is much the same as stage three treatments for mania. We can
keep the patient on the medications that work for them on stage two as a
maintenance treatment. We need to continue to monitor any side effects and
check laboratory results periodically to make sure the patient’s liver function and
kidney function are within normal range. If side effects are significant,
typically there will be either a dosage adjustment or possibly a change to
another medication. If side effects are mild to moderate and tolerable, but
there is only partial improvement in symptoms after several weeks of treatment,
then a decision will be made to either change to a different medication or to
add another medication (i.e., augmentation). Augmentation medication usually is
chosen from a different class of mood stabilizers.
In conclusion, there is an important reason for emphasizing the process in prescribing these medications.
Many times, people being treated for bipolar illness or their family members
become worried as they begin to encounter side effects, or they must go through
what seems like an endless number of laboratory tests or changes in medications
or medication doses. Many people become concerned that these medication changes
suggest that their doctor may not be competent or that their case of bipolar is
especially treatment-resistant. This then can lead to discouragement and
feelings of pessimism. Here is the truth: the pathway to recovery and good
outcomes, more often than not, is complicated. The rule, not the exception, is
that people will be tried on several if not many medications in the search for
the right drug or medication combinations. It is so important to help patients
understand this and not conclude that the frequent changes in medications are
necessarily a reason for concern. The fact is that bipolar disorder is
challenging to treat and often requires a considerable amount of time
systematically trying various medications before the right medication
combinations are found.
Adjunctive Therapies for Bipolar Spectrum Disorder
Bright light therapy (using a commercially available light box which generates 10,000
lux of light intensity for 10-30 minutes a day) has been used for treating
bipolar depression, especially for those who routinely have winter depressions
or who work the night shift. This treatment is typically combined with
medication treatments and, like all treatments for depression, it too carries a
risk of provoking mania in people with bipolar disorder.
Omega-3 Fatty Acids Approximately 60% of the human brain is composed of lipids (fats)
and 30%-35% of brain mass is made up of omega-3 fatty acids. These molecules
are important in forming cell membranes and synapses and in facilitating nerve-cell
actions. The most abundant dietary source of omega-3 fatty acids is fish and shellfish.
In cross-cultural studies it has been found that in countries where people eat
a lot of fish and other seafood, the severity of mood disorders is less. This
interesting finding led researchers to carefully evaluate the impact of diet on
mood. During the past ten years, a number of studies have been conducted
with people suffering from bipolar disorder and also major depression.
Unfortunately, omega-3 fatty acids have not been shown to be effective as an
augmenter to standard mood stabilizers. However, people with bipolar disorder
suffer from high rates of heart disease and strokes, and thus omega-3, which
can afford some protection against vascular disease, may be helpful in that
respect.
Exercise therapy has recently been shown to be effective in treating major
unipolar depression, however, to date there are no studies of this approach in
treating bipolar depression.
Psychotherapy
Although medication treatment is the backbone of successful
therapy for bipolar disorder, a number of studies have clearly shown that
psychotherapy, especially Interpersonal and social rhythm therapy,
Cognitive-behavioral therapy, or Family-based Psychoeducational therapy, can
significantly contribute to better-treated outcomes.
Anxiety Disorders
General Considerations
Anxiety disorders are the most common mental disorders in the U.S. They affect 40 million adults every year, that is about 19.1% of the population age 18 and older. An estimated 31.1% of U.S. adults experience an anxiety disorder at some time in their lives and many become chronic conditions. Clinical studies have shown that the combination of psychotherapy and medication treatment is the most effective treatment. Almost all anxiety disorders discussed in this section have been found to respond well to cognitive behavioral therapy (CBT) and/or acceptance and commitment therapy (ACT), or to exposure-based cognitive therapy. Despite this, medication treatments are often useful and even necessary as the first-line of treatment, owing to the following:
- In many communities there may not be professionals trained to provide therapy.
- Some patients refuse therapy owing to the fact that such treatments require exposure, and people are too afraid to undertake the treatment.
- Often, symptoms are so debilitating that medication intervention is an important first step to take to reduce suffering and help people re-engage in normal life functioning and then begin psychological treatments.
- Some patients with marginal ego strength may not be able to tolerate therapy well.
One caveat regarding using medication initially is that If at some point exposure-based treatments are employed, it will be necessary to gradually reduce the dose of medication so that the client experiences some anxiety. This is required since it has been found that exposure therapy and deconditioning are not successful unless the person experiences some amount of anxiety, is able to undergo exposure trials using anxiety-management skills, and has the experience of enduring anxiety without catastrophe. This is essential in order to experience mastery.
Diagnostic Issues
The following disorders have been classified in DSM-5-TR as
anxiety disorders:
- Generalized Anxiety
Disorder: chronic, persistent, and excessive anxiety and worry about various
domains, including work and school performance, with difficulty controlling it for at least six months.
- Panic Disorder: characterized
by repeated and unexpected panic attacks that erupt and rapidly escalate into
intense anxiety within minutes with physical and/or cognitive symptoms over a one-month
period. Often accompanied by anticipatory anxiety and phobic avoidance (e.g.,
agoraphobia).
- Social Anxiety:
anxiety symptoms are only experienced in social interactions and situations
because of a fear of being scrutinized. It typically lasts for six months or
more.
- Agoraphobia: fearful
and anxious in many different situations and thoughts of escaping might be
difficult or help might not be available.
- Substance/Medication-Induced
Anxiety Disorder: anxiety is caused by substance intoxication or withdrawal or a
medication treatment. Of note here is that the use of caffeine can cause or
significantly exacerbate anxiety symptoms. In every case presenting with
anxiety, it is critical to evaluate the presence of caffeine use. The caffeine questionnaire
at the end of this course can be helpful in screening for caffeine use.
- Anxiety Disorder Due
to Another Medical Condition: anxiety is due to the direct pathophysiological
consequence of another medical condition.
- Specific Phobia:
fearful or anxious about, or avoidant of, circumscribed objects or situations.
- Separation Anxiety
Disorder: when feeling fearful or anxious about being separated from attachment
figures is out of the normal range and is developmentally inappropriate.
- Selective Mutism: a
consistent failure to speak in social situations even though this person can speak
more comfortably in other situations.
- Other Specified
Anxiety Disorder or Unspecified Anxiety Disorder: anxiety symptoms do not meet
the full criteria of above anxiety disorders but still cause clinically
significant distress or impairment in the person’s life.
Psychopharmacology For Anxiety Disorders
There are two major classes of medications used to treat anxiety disorders: antidepressants (all of those mentioned above with the exception of Wellbutrin, which can increase anxiety in some patients) and the anxiolytics (commonly referred to by their class name: benzodiazepines., formerly known as tranquilizers ). We will also consider adjunctive and experimental treatments. We will focus on the medication treatments for generalized anxiety disorder, panic disorder, and social anxiety.
Antidepressants have been addressed in detail above in depression
treatment, thus there will be only a few issues presented here. Most of the
antidepressants, especially those that target serotonin and norepinephrine,
have been found to be highly effective in treating most forms of anxiety
disorders. However, all have the tendency to produce initial
activation (affecting 10%-20% of people starting these medications). This
is a side effect of the drugs that can appear within a few hours after taking
the first dose. This is very problematic for those with anxiety disorders. The
increase in anxiety that occurs due to activation ranges from mild to marked
and typically lasts for ten days, and then begins to subside (although in
some cases it may persist for a longer period of time). Most patients who
encounter this become frightened by the increase in anxiety and stop taking the
antidepressant. Thus, we need to start antidepressants at a very low dosage and
slowly titrate it up as the patient can tolerate it. Another popular and effective
way to address this issue is to co-administer a benzodiazepine. Using a
benzodiazepine at the low dosage in short duration is considered to be appropriate
treatment as an adjunctive treatment. If it is dosed appropriately, it begins
to reduce anxiety within 30-45 minutes and can also operate to override
activation. The common consensus is to use a benzodiazepine for the first month
of treatment and then to taper it off. After one month of treatment, most
antidepressants begin to show anti-anxiety effects. There is one note of
caution: Since benzodiazepines can be habit-forming, they should be used with
great caution in patients who have a history of alcohol or other substance
abuse. In such individuals, an alternative to the use of a benzodiazepine is
hydroxyzine (brand names: Atarax; Vistaril). This is an antihistamine that has
significant antianxiety properties. Benadryl can also be used to treat
initial insomnia. Another option is to use beta-blockers such as propranolol (Inderal)
to manage physical symptoms of anxiety.
Benzodiazepines
Generic Name |
Brand Name |
Typical Adult Daily Dose |
Diazepam |
Valium |
2.5-10 mg |
Clonazepam |
Klonopin |
0.5-4
mg |
Lorazepam |
Ativan |
2-10
mg |
Alprazolam |
Xanax |
0.25-4
mg |
Medications used to treat anxiety-related initial insomnia:
(See also, the course Bipolar Spectrum Disorders: Diagnosis and Pharmacologic
Treatment.)
Generic Name |
Brand Name |
Typical Adult Nighttime Dose |
Temazepam |
Restoril |
15-30 mg |
Triazolam |
Halcion |
0.125-0.5
mg |
Zolpidem |
Ambien |
5-10
mg |
Zaleplon |
Sonata |
5-20
mg |
eszopiclone |
Lunesta |
1-3
mg |
diphenhydramine |
Benadryl |
25-50 mg |
Ramelteon |
Rozerem |
8 mg |
survorexant |
Belsomra |
10-20 mg |
Doxepin |
Silenor |
3-6
mg |
Special Concerns: If benzodiazepines are being taken on a regular
basis, the body develops a tolerance for the medication. When this happens,
typically the drugs continue to work to reduce anxiety, but the problem is that
when there is tolerance, if one abruptly stops the medication there can be
withdrawal symptoms. Withdrawal symptoms usually include nervousness,
agitation, difficulty falling to sleep, and on occasion can produce seizures.
This needs to be taken very seriously. If a patient has been taking a
benzodiazepine on a daily basis for more than six weeks, and especially if the
dose is moderate-to-high, withdrawal reactions are a very real risk. One should
never abruptly stop taking the medication without first consulting with their physician. It is also a good idea to be especially careful to monitor the
supply of the medications so that refills can be requested in a timely fashion.
Many people find it helpful to keep at least a two-day supply on hand in the
event that it takes longer than usual for a prescription to be refilled.
Additionally, benzodiazepines can be abused and should not be prescribed to
people with a history of substance use disorder.
The onset of action of benzodiazepines is 30-45 minutes. As noted
above, most antidepressants require a minimum of four weeks of treatment before
the first signs of clinical improvement are noted.
Generalized Anxiety Disorder (GAD)
Selecting antidepressants to treat GAD is based on the treatment guideline
and the individual’s history and conditions. The first-line treatment is
selective serotonin-reuptake inhibitor (SSRI), the second-line treatment is
serotonin-norepinephrine reuptake inhibitor (SNRI). For the third-line
medication we can use buspirone (BuSpar). (Please see Depression treatment on
SSRIs and SNRIs.) There are other medications that can be used to treat anxiety
disorders, such as mirtazapine (Remeron), gabapentin (Neurontin), and pregabalin
(Lyrica).
Antidepressants (SSRIs and SNRIs) are generally very effective in treating
GAD. It usually requires four to six weeks of treatment, or longer in some
instances, before patients begin to show clinical improvement. We need to
inform patients about this so they can understand and not prejudge that the
medication does not work. The doses used to treat GAD are similar to those used
to treat major depression.
Buspirone (BuSpar) dosage starts with 7.5mg twice daily initially and can gradually
increase up to 60 mg/daily. The symptomatic improvement is seen to emerge
gradually, and primarily is experienced by the patient as a decrease in worry
and rumination. BuSpar has a very favorable side-effect profile and is well
tolerated, however, at this time, its efficacy is not as robust as that seen with benzodiazepines
or antidepressants.
Pregabalin (Lyrica) dosage is at 75mg twice daily initially and can increase to
higher dosages, up to 600 mg/day, and Gabapentin (Neurontin) can take
between 300 mg to 2400 mg/day. Both have significant antianxiety effects and have
been found to be useful in the treatment of generalized and social anxiety
disorder. However, patients can develop tolerance and can have withdrawal
symptoms if they stop taking it suddenly. Clinically, it has never been
used as the first-line treatment for anxiety disorder.
Panic Disorder
Two classes of medications are effective in treating panic
disorder: antidepressants and benzodiazepines. At the initial stage, we can use
both classes of medication; when the patient’s panic attack subsides, the patient can continue with an antidepressant.
For antidepressants, we use SSRIs and SNRIs, even TCAs. Please see
the Depression section regarding medication selection.
The advantage to using benzodiazepines is the quick onset of
action. This can be a godsend for patients suffering frequent and severe panic
attacks. The particular drugs used most often are the high-potency
benzodiazepines: Xanax, Ativan, and Klonopin (other benzodiazepines often cause
too much sedation). Doses required to contain panic attacks vary considerably
and in every case the patient is started on a low dose and gradually increased
until symptom control is achieved. These medications have a rapid onset of action
and are generally well tolerated. The preference for using benzodiazepines is
in its scheduled, longer-acting agents so that medication use is time-dependent
rather than response/panic-dependent. It is necessary to have the medication in
the body continuously, 24 hours per day, to avoid panic attacks (i.e., they
cannot be taken only on an “as needed” or PRN basis since panic attacks come on
suddenly and the drugs require 30-45 minutes to become active in the central
nervous system). Once panic symptoms have been eliminated or greatly reduced
with antidepressants, we can taper off benzodiazepine.
Social Anxiety
Antidepressants such as SSRIs and SNRIs can be considered as the first-line treatment. Monoamine oxidase inhibitors (MAOI’s) can be used to treat severe and pervasive forms of social anxiety that have not responded to SSRIs or SNRIs. We still can use benzodiazepineson a short-term basisto manage symptoms. Another treatment choice is to use propranolol (Inderal), a beta blocker, at 30 minutes to one hour before exposure to a social situation at doses of 10mg-40 mg.
Herbs and over-the-counter supplements
There are many herbs and over-the-counter supplements that have shown some anti-anxiety benefits, but the majority of them have insufficient evidence for reducing anxiety symptoms. Some of them can cause liver damage if used excessively, such as Kava Kava. People still use them experimentally to relieve their anxiety symptoms. The commonly used herbs are chamomile tea, passion flower, valerian, lavender, and peppermint. Over-the-counter supplements include 5-HTP, ashwagandha, magnesium, theanine.
Coffee and anxiety
For healthy adults, the FDA has cited 400 milligrams a day(that’sabout four or five cups of coffee a day), as an amount not generally associated with dangerous, negative effects. However, when anxiety is a prominent feature, then caffeine use should be completely avoided. Many individuals who suffer from anxiety disorders inadvertently consume significant amounts of caffeine and this always complicates psychological or medical treatment. “Energy drinks” should also be avoided. Caffeine content of energy drinks vary. There is a report that drinking more than 250 mgs a day, if taken after noon, may interfere with deep sleep. If caffeine use exceeds 250 mg. per day, there is a likelihood that it will interfere with slow wave/deep sleep and may play a role in increasing any psychiatric symptoms. Please see Appendix ? for the caffeine questionnaire to know how much caffeine is in various drinks.
| CAFFEINE CONSUMPTION
QUESTIONNAIRE |
| |
|
|
Average number of servings/doses/tablets per day |
|
Average total per day |
Beverages |
Coffee
(6 oz.) |
125
mg |
X |
|
= |
|
Espresso
(one oz.) |
50
mg |
X |
|
= |
|
Decaf
Coffee (6 oz.) |
5
mg |
X |
|
= |
|
Tea
(6 oz.) Black |
50
mg |
X |
|
= |
|
Green
Tea (6 oz.) |
35
mg |
X |
|
= |
|
Energy
Drinks |
200
mg (equivalent*) |
|
|
|
|
Cocoa
(6 oz.) |
15
mg |
X |
|
= |
|
Caffeinated
Soft Drinks (12 oz.) |
40-60
mg |
X |
|
= |
|
Chocolate
Candy Bar |
20
mg |
X |
|
= |
|
Over-the-Counter
Medications |
Anacin |
32
mg |
X |
|
= |
|
Appetite-Control
Pills |
100-200
mg |
X |
|
= |
|
Dristan |
16
mg |
X |
|
= |
|
Excedrin |
65
mg |
X |
|
= |
|
Midol |
132
mg |
X |
|
= |
|
NoDoz |
200
mg |
X |
|
= |
|
Triaminicin |
30
mg |
X |
|
= |
|
Vanquish |
33
mg |
X |
|
= |
|
Vivarin |
200
mg |
X |
|
= |
|
Prescription
Medications |
Cafergot |
100
mg |
X |
|
= |
|
Fiorinal |
40
mg |
X |
|
= |
|
TOTAL
MG. CAFFEINE PER DAY |
|
*Caffeine content of energy drinks vary.
Attention Deficit/Hyperactivity Disorder (ADHD)
Introduction
ADHD occurs worldwide in about 7.2% of children and 2.5% of adults
in a cross-national meta-analysis (DSM-5-TR). With neurological maturation,
most teenagers with ADHD will experience a noticeable reduction in motoric
restlessness/hyperactivity, but the core symptoms of ADHD (e.g., impulsivity,
impaired attention, and lack of intrinsic motivation) continue through
adolescence and on into adulthood. Most experts agree that about one-third of
ADHD children completely outgrow the disorder by early adulthood (likely due to
the ongoing maturation of the prefrontal lobes which may continue until the
late 20s or early 30s). Two-thirds experience ongoing symptoms throughout life.
Diagnostic Issues
It is very important to emphasize that most psychiatric disorders
in childhood present with some degree of motoric restlessness and inattention. Delays
in language, motor, or social development are not specific to ADHD, but often
co-occur. Emotional dysregulation or emotional impulsivity commonly occurs in
children and adults with ADHD (DSM-5-TR). These outwardly observable behaviors
absolutely do not automatically lead to a diagnosis of ADHD. The box below
lists those disorders that must be considered in any comprehensive evaluation
of children or adolescents with hyperactivity/impulsivity and inattention.
Differential
Diagnosis of Childhood Onset Psychiatric Disorders Presenting with
Hyperactivity/Impulsivity and Inattention |
- Diffuse brain damage (e.g.,
commonly seen in fetal alcohol syndrome or fetal exposure to other drugs
of abuse)
- Post-traumatic brain injuries
- Reactive attachment disorders
- Anxiety disorders (e.g., separation
anxiety)
- Post-traumatic stress disorder
- Oppositional defiant disorder
- Intermittent explosive disorder
- Specific learning disorder
- Disruptive mood dysregulation disorder
- Substance use disorder
- Situational stress
- Bipolar mania
- Pre-psychotic conditions
- Impaired affect regulation
associated with severe early abuse or neglect
- Boredom (especially likely in
bright children who are academically under-stimulated)
|
The diagnosis of ADHD is based largely on three sources of data:
family history (since ADHD is considered to be a genetically transmitted
disorder and thus often runs in families. The heritability of ADHD is
approximately 74%), a very careful history detailing the nature and onset of
behavioral symptoms, and a description of current symptoms (especially as they
vary across situations, i.e., home, school). It is also a diagnosis of
exclusion (one must always first rule out those disorders listed above).
The most common presentation for ADHD is an early onset (often
present in infancy) of restlessness, unstable sleep patterns, affective
lability (especially, crying a lot and difficulty in being soothed). Most true
ADHD children are identified in preschool when they have their first sustained
contact with other children and encounter social standards (expectations to
control behavior, follow rules, and stay on task in age-appropriate ways).
There is some controversy among experts, but general agreement is that
this very early onset of significant behavioral problems is very
characteristic of ADHD.ADHD cannot be diagnosed in the absence of any symptoms
prior to age 12. However, there is emerging data to suggest that some children
destined to have bipolar disorder may show early-onset behaviors that are
ADHD-like (i.e., prodromal symptoms of bipolar disorder).
During childhood, the following symptoms predominate:
hyperactivity, impulsivity, impaired self-control, difficulties staying on task,
and limited ability for intrinsic motivation (e.g., motivation to stay focused,
especially on mundane, non-exciting, or low stimulus-value tasks). Such
symptoms are often highly context-dependent; that is, most noticeable in
situations requiring that the child remain still and quiet (e.g., in the
classroom), yet may not be as noticeable when in an environment that is
inherently exciting, novel, or stimulating (e.g., playing video games).
With adolescence, as noted above, motoric hyperactivity often is
reduced, but core symptoms remain. Disorganization (manifested by messy
lockers, notebooks, and bedrooms) often is pronounced in the adolescent ADHD patient
(also true with adults suffering with ADHD), as are increasing problems
adapting to society’s and school’s demands for independent task performance and
self-control.
Neurobiology
Numerous studies suggest impaired frontal lobe functioning in
people suffering from ADHD (evident in studies of metabolic functioning: e.g.,
SPECT and PET scans). In addition, abnormalities have been shown in the
dopamine neurotransmitter system. Likewise with dopamine agonists (e.g.,
stimulants or bupropion). Minor structural abnormalities have also been found
in the brains of ADHD subjects (e.g. smaller cerebellar volumes, smaller
volumes of frontal and temporal areas, and a smaller caudate nucleus).
No biological marker has been found for diagnosing ADHD, although ADHD
has been associated with elevated power of slow waves (4-7Hz “theta”) as well
as decreased power of fast waves (14-30Hz “beta”). A later review found no
differences in theta or beta power in either children or adults with ADHD
related to control subjects. (DSM-5-TR)
Appropriate treatment with stimulants may not only reduce
symptoms, but also may also normalize the chemical microenvironment of the
developing brain, and ensure more normal brain maturation. Castellanos, et al.
(2002) demonstrated that ADHD children have smaller cerebral and cerebellar
volumes than age-matched controls. The degree of reduction in frontal,
temporal, cerebellar, and white matter volumes correlated significantly with
parent and teacher ratings of ADHD symptom severity. Unmedicated ADHD subjects
exhibited strikingly smaller white matter volumes compared to both controls and
medicated ADHD children (treated with stimulants). This suggests that
appropriate treatment may be neuroprotective.
Psychopharmacology of ADHD
There are two classes of medications with empirical support of
efficacy in the treatment of ADHD: stimulants and non-stimulants. We also use
some antidepressants to treat ADHD as an off-label treatment, i.e., Wellbutrin.
Stimulants
There are two types of stimulants. One is amphetamines and the other
is called methylphenidates. The mechanism of action of stimulants is inhibition
of dopamine reuptake (additionally, amphetamines promote increased release of
dopamine from vesicles). Each group has different formulary medications based
on the onset of action or duration of action, with symptom reduction occurring
30-45 minutes after ingestion, or a duration of action ranging from 4-12 hours,
i.e., short-acting or long-acting. Each stimulant group has a liquid formula, a
patch formula, and a chewable tablet as well to fit different needs. Listed
below are the currently available stimulants. Depending on the formulation,
dosing is generally two to three times daily, with some long-acting products
providing once-daily dosing. What is most important is to find the best
possible dose and dosing schedule for the patient.
In the past few years there has been an explosion of various drugs
that have received FDA approval, both on formulations for amphetamine and
methylphenidate. Listed below are those in most commonly in use. The dosage
shown is only for adult dosage, not for children or adolescents.
Stimulants |
Amphetamine Formulations |
Short-Acting |
Long-Acting |
Evekeo/EvekeoODT (d-& l-amphetamine sulfate) |
5-40 mg/day (1-2x/day) |
Dyanavel XR (d-&I-amphetamine sulfate) |
2.5-20 mg/day |
Zenzedi (dextroamphetamine sulfate) |
5-40 mg/day (1- 2x/day) |
Mydayis (mixed amphetamine salts) |
12.5-50 mg/day |
Adderall (mixed amphetamine salts) |
5-40 mg/day (1- 2x/day) |
Adzenys XR-ODT/ER (d-&I-amphetamine) |
12.5 mg/day |
ProCentra (dextroamphetamine sulfate) in solution |
5-40 mg/day (1- 2x/day) |
Adderall XR (mixed amphetamine salts) |
5-60 mg/day |
| |
|
Dexedrine Spansule (d-amphetamine sulfate) |
10-60 mg/day (1- 2x/day) |
| |
|
Vyvanse (lisdexamfetamine) in capsule and chewable |
10-70 mg/day |
Methylphenidate Formulations |
Short-Acting |
Long-Acting |
Focalin (dexmethylphenidate) |
5-20 mg/day, divided twice a day |
Concerta |
18-72 mg/day |
Ritalin |
10-60 mg/day divided BID or TID |
Aptensio XR |
10-60 mg/day |
Methlphenidate chewable |
10-60 mg/day divided BID or TID |
Focalin XR (dexmethylphenidate) |
5-30 mg/day |
Methylin Solution |
10-60 mg/day divided BID or TID |
Quillivant XR (liquid formula) |
20-60 mg/day in 25 mg/5mL (5mg/ml) |
| |
|
Ritalin LA |
20-60 mg/day |
| |
|
Metadate ER |
20-60 mg/day |
| |
|
Daytrana (path) |
10-30 mg/day (9h on and 15h off) |
| |
|
Azstarys (dexmethylphenidate + serdexmethylphenidate) |
39.2/7.8-52.3/10.4 mg/day |
| |
|
Jornay PM |
20-100 mg/night (dosed in the evening) |
There are more than 200 well-controlled studies of stimulant use
and the outcomes are significantly positive. Assuming an accurate diagnosis,
any one stimulant taken results in approximately 70% response rate (good to
very good response). Although the stimulants are similar, there are
differences. Thus, if a trial with one stimulant (e.g., methylphenidate) is
less than optimal, then it is advisable to conduct a trial on another stimulant
(e.g., dextroamphetamine). If systematic trials are conducted on each of the
three classes of stimulants, good outcomes are seen in about 90% of patients
treated (Barkley, 2000). Across studies, effect sizes are quite high, ranging
from 0.8 to 1.0.
The following briefly highlights important issues regarding
stimulant treatment:
- Very low risk of abuse
in ADHD patients.
- Side effects
- Initial insomnia (if taken later in the day)
- Solutions - earlier dosing, clonidine or trazodone given at
bedtime
- Anorexia generally only affects the patient when the drug
is active (i.e., usually only interferes with appetite at lunchtime). Has not
been associated with significant problems obtaining adequate nutrition. Focalin
may have less of this effect.
- Stomach ache
Mild dysphoria
- Switch classes of stimulants
- Add an antidepressant such as bupropion
- Lethargy, sedation or impaired concentration - usually
indicates that the dose is too high
- ADHD affects all
aspects of life, not just academic performance. Therefore, it is often best to
administer medications every day (i.e., not just on school days).
- Start treatment with
immediate-release formulations and then switch to extended-release preparations
if tolerated and effective.
- Stimulants only work
for a short period of time and the positive effects wear off in the late
afternoon or evening. Thus, co-administration of antidepressants (see below)
may be an option for targeting symptoms later in the day and/or adding a short-acting
stimulant in the afternoon.
- Most children successfully
treated with stimulants will require ongoing medication treatment well into
adolescence and possibly into adulthood.
Failure to accurately diagnose and then to mistreat with
stimulants can have very adverse consequences (see table below).
Consequences
of Misdiagnosis and Stimulant Treatment |
Diagnosis |
Consequences |
Anxiety disorder |
Increased anxiety |
Agitated depression |
Increased agitation |
Pre-schizophrenic |
Psychosis |
Bipolar disorder |
Increased manic symptoms
may cause cycle acceleration |
Situational stress |
Failure to address psychological issues |
Non-stimulants include four drugs that have been approved by the FDA.
Two of them are Alpha-2 Adrenergic Agonists. They have been
approved for ADHD treatment for ages 6-17 years old.
Clonidine extended release (Kapvay), and guanfacine extended
release (Intuniv) may be used to treat core ADHD symptoms (see figure below),
however they are most effective in reducing irritability, aggression, and
impulsivity, and in promoting sedation (to treat initial insomnia). Alpha-2
agonists are also the treatment of choice for comorbidity of tics.
Alpha-2
Adrenergic Agonists |
Generic Name |
Brand Name |
Typical Doses |
clonidine ER |
Kapray |
0.1-0.4 mg/day (1) |
guanfacine ER |
Intuniv |
1-7 mg (2) |
(1) Take daily or divided twice a day
(2) Daily based on the weight, and the dosage is different |
There have been 25 reported cases of death in children taking
clonidine in conjunction with a stimulant (U.S. and Canadian data
combined). However, the FDA has conducted an investigation and failed to find
any significant cause for concern in co-administering these drugs. The deaths
seen, as determined upon autopsy, occurred in children who had evidence of pre-existing,
yet un-diagnosed significant cardiac disease. It is prudent to do cardiac
screening on all subjects prior to administering the medications, including a
family history of early heart disease, sudden infant death syndrome, a history
of fetal alcohol exposure, or any of the following symptoms: sudden,
unexplained loss of consciousness; dizziness; tachycardia; or chest pain.
Should any of these exist, it is recommended that the child be given a
pre-treatment EKG. Combined use of Alpha-2 agonists and stimulants is a common
practice (both for treating ADHD and comorbid ADHD and tics) (Walkup, 2003).
For adults, we also need to ask their medical history and family of history of
cardiac disease. ECT may be necessary as well prior to the medication treatment.
Another two non-stimulants are selective norepinephrine reuptake
inhibitors
20% of ADHD children will experience co-occurring depression and
anxiety. Antidepressants certainly may be helpful in reducing mood symptoms.
However, beyond this use of antidepressants, certain classes of antidepressants
have been shown to have positive effects on core ADHD symptoms. Not all
antidepressants treat ADHD; only those that increase the availability of
dopamine or norepinephrine (thus SSRIs, although often a good adjunct for
treating anxiety or depression, are not effective in treating core ADHD
symptoms). The FDA has approved two selective norepinephrine reuptake
inhibitors for ADHD in children and adolescents and adults. See the list below.
Antidepressants
Used to Treat ADHD |
Generic Name |
Brand Name |
Typical Daily Dosage |
atomoxetine |
Strattera |
40-100 mg |
viloxazine |
Qelbree |
100-600 mg |
Treatment outcomes with antidepressants are not as robust as that
seen with stimulants, however, they afford several advantages:
- It is not a control
medication;
- Effects (generally
seen within one to four weeks after initiating treatment) typically last
24-hours-a-day; and
- Can treat comorbid anxiety
and depressive mood.
Appendix I
Psychiatric Medications: Quick Reference Guide
To keep up-to-date with new medications, click on the website www.Rxlist.com for a free download of the Psychiatric Medications Quick Reference
Chart.
Note: To the best of our knowledge doses and side effects listed
in the Quick Reference Guide, linked above, are accurate. However, this is
meant as a general reference only and should not serve as a guideline for
prescribing medications. Please check the manufacturer’s product information
sheet or the PDR (Physician’s Desk Reference) for any changes in dosage
schedule or contraindications. (Brand names are registered trademarks.)
Mood Stabilizers/Anti-manic Agents
Lithium
Lithium Facts:
- the drug with the best
track record in preventing recurrences of manic episodes;
- lithium is a naturally occurring alkali metal; and
- lithium is one of two
psychiatric drugs proven to substantially reduce the incidence of suicide.
Brand Names:
- Lithium carbonate: Eskalith,
Eskalith CR, Lithostat tablets, Lithobid slow-released tablets, Lithium
carbonate tablets.
- Lithium citrate:
Lithium citrate syrup.
Uses:
- Treats mania and
bipolar depression and is used to reduce recurrences of mania and depression.
- Used to augment
antidepressants in treating major depression
Typical Adult Daily Doses (Eskalith or Lithobid, most commonly
prescribed):
Note: What matters with lithium treatment is not the dose, per se,
but the blood level (which is carefully monitored). A lithium level between 0.8
and 1.2 mEq/L (mEq/L is the technical designation for what is commonly called
the lithium level) is generally felt to be in the therapeutic range for
treating mania. Once the manic episode is resolved, then it is common practice
to lower the dose to establish a blood level somewhere between 0.6 and 0.8 mEq/L.
Blood levels above 1.2 are associated with significant side effects, and levels
above 2.0 can be dangerous.
Onset of effects (how long it takes to start working):
Laboratory Tests:
Prior to starting treatment with some medications, laboratory tests are
required to establish baseline measures of the functioning of certain bodily
systems. The following are typically required prior to starting treatment (and
those with an * will need to be monitored periodically during treatment):
ECG (EKG)*, electrolytes, complete blood count, kidney function
tests* (BUN, creatinine, urinalysis), thyroid tests*, calcium*, pregnancy test (for
reproductive-age females).
Laboratory Tests Routinely Done On An Ongoing Basis:
Those tests flagged above with an asterisk are repeated periodically. In
addition, it is necessary to periodically check lithium blood levels. This is
done frequently during the first weeks of treatment and when there are
significant changes in dosage. Initial monitoring: Every one to two weeks until
desired serum concentration is achieved, then every two to three months for the
first six months. Stable monitoring is every 6 to 12 months.
Lithium can cause weight gain, so we need to monitor body mass
index (BMI).
Common Side Effects:
- Hand tremor or muscle
weakness
- Lethargy, drowsiness (may impair
the ability to safely drive an automobile)
- Increased thirst and
frequency of urination
Less Common Side Effects (these should be reported to prescribing physician immediately):
Rare or potentially dangerous side effects (If these occur, immediately contact
prescribing physician):
- Soreness of mouth,
throat, or gums
- Swelling of the neck
or face
- Severe nausea,
vomiting, weakness, fever, flu-like symptoms
- Marked increase in
thirst and very frequent urination
Habit-forming/Addiction Potential:
Interactions with other medications:
Here are only the most common medications with which the drug may
cause adverse interactions:
- Non-steroidal
anti-inflammatory pain medications such as ibuprofen
Safety during pregnancy:
- A rare birth defect
(Ebstein’s anomaly, a heart defect) if taken during the first trimester. This
occurs in 0.1%-0.2% of fetuses exposed to lithium. If the patient is planning
to get pregnant or suspects that she may be pregnant, contact prescribing physician.
Breast-feeding:
- Not recommended when
taking lithium.
Special Concerns:
- Lithium is a very dangerous drug if taken in either an accidental or
intentional overdose. We call it “lithium toxicity” and it is a
life-threatening event, so seek immediate medical attention.
Appendix II
Anticonvulsant Mood Stabilizers
Anticonvulsant Facts:
Anticonvulsants are medications originally developed to treat
epilepsy. It was only by accident that it was discovered that some
anticonvulsants also have the ability to treat mania. In addition, the
anticonvulsant Lamictal has antidepressant actions and can be used to treat
bipolar depressive episodes.
| Generic
Name
|
Brand Name |
Typical Adult Daily Dose |
Divalproex sodium |
Depakote |
750- 1500 mg |
Carbamazepine |
Tegretol, Equetro |
800-1600 mg |
Oxcarbazepine (off-label) |
Trileptal |
600mg-1200 mg |
Lamotrigine |
Lamictal |
50-200 mg |
Uses:
Treat mania. Lamictal
is used to treat bipolar depression. Research evaluating the ability for
anticonvulsants to help prevent recurrences of mania and bipolar depression is
not yet conclusive. Depakote, Trileptal and Tegretol likely help to prevent
recurrences of mania and Lamictal likely reduces the recurrence of bipolar
depression.
Therapeutic Blood Levels:
Two of the
anticonvulsant mood stabilizers must be periodically monitored to check the
levels of medication present in blood.
Depakote blood levels: |
50-125 mcg/ml |
Tegretol blood levels: |
4-12 mcg/ml |
Trileptal blood levels: |
not yet established |
Lamictal blood levels: |
not necessary to monitor |
Onset of effects:
Generally, 7-10 days
(one exception: if high doses of Depakote are administered, the loading dosage
is 50 mg/kg and the effects can be seen in less than five days)
Laboratory Tests:
Required for Depakote, Tegretol, and Trileptal. Specific tests depend on which
drug is used, but often include:
Complete blood count, platelets, electrolytes, cholesterol,
triglycerides, sonogram of ovaries (optional for females under the age of 20
treated with Depakote), liver function tests, ECG (EKG), pregnancy test. For
Topamax, kidney function tests (BUN and creatinine).
Pretreatment labs are generally not required for Neurontin or
Lamictal.
Laboratory Tests routinely done on an ongoing basis:
Tegretol and Depakote blood levels must be monitored (especially during the
initial weeks of treatment). Generally, once a person is stabilized on
Depakote, blood level monitoring is not necessary. However, those treated with
Tegretol must have periodic and ongoing monitoring of Tegretol levels.
Ongoing lab tests are generally not required for Lamictal.
Common Side Effects (each medication has specific side effects, however listed
here are side effects that can be seen in most of the anticonvulsants):
- Unsteadiness when
standing or walking
- Changes in menstrual
cycle
Less Common Side Effects (should be reported to prescribing physician):
- Infertility problems
(seen with some women under the age of 20 treated with Depakote), also
accompanied by menstrual irregularities.
- Changes in hair: hair
loss (usually transient) or changes in hair texture
Rare or potentially dangerous side effects (if these occur,
patient should seek emergency medical care):
- Skin rash (mild rashes
are fairly common, but a rash that is severe and rapidly increases in severity
should be reported to a doctor immediately)
- Yellow tint to skin or
eyes
- Severe problems with
balance and dizziness
- Soreness of mouth,
throat, or gums
- Easily bleeding or
bruising
- Severe nausea,
vomiting, and abdominal pain
Habit-Forming/Addiction Potential:
Interactions with other medicines (varies depending on the specific
drug):
- Birth control pills
(especially with Tegretol)
- Anticoagulants (blood
thinners)
- Aspirin (moderate to
high doses)
- Antibiotics
(especially with Tegretol)
- Propoxyphene (Darvon)
(especially with Tegretol)
- Antidepressants
(Anticonvulsant mood stabilizers are often prescribed for patients who are also
taking antidepressants. Generally, there are no problems unless the
antidepressant doses are high)
- Antacids may affect
the absorption of some anticonvulsants
Safety during pregnancy:
There is a risk of
birth defects when taking anticonvulsant mood stabilizers during pregnancy
(especially in the first trimester; significant birth defects seen most
commonly in treatment with Depakote). Lamictal is a relatively safe medication
during the pregnancy.
Breast-feeding:
Not recommended when
taking anticonvulsants.
Antipsychotic Medications
Facts:
Antipsychotic medications were first developed to treat psychotic symptoms such
as hallucinations and thought distortion, mainly for schizophrenia and
schizoaffective disorder. The first such drugs were found to be effective in
reducing psychotic symptoms, but they were notoriously “dirty” drugs, causing
significant side effects. Since the mid-1990s, new and improved antipsychotics
have been developed and marketed. These newer-generation medications are not
side effect-free, but they are considerably safer and better tolerated. The
newer drugs are commonly referred to as atypical antipsychotics or
second-generation antipsychotics (SGAs).
Although atypical antipsychotic medications are highly effective
in treating psychotic symptoms, it has been found that they are also good
treatments for mania and possibly for mood stabilization in general. They also
have a role in treating aggression and agitation. Thus, these medications are
currently being widely used to treat bipolar disorder even in individuals who have
no psychotic symptoms.
Atypical Antipsychotic Medications: Generic and Brand Names and Typical
Adult Daily Doses
Generic Name |
Brand Name |
Typical Adult Daily Dose |
olanzapine |
Zyprexa |
5-20 mg |
risperidone |
Risperdal |
2-6 mg |
quetiapine |
Seroquel |
200-800 mg for bipolar I disorder, manic
and 300 mg for bipolar depression |
ziprasidone |
Geodon |
40-160 mg with food |
aripiprazole |
Abilify |
15-30 mg |
asenapine |
Saphris |
5-20
mg |
lurasidone |
Latuda |
20-120
mg with food |
cariprazine |
Vraylar |
3-6
mg |
Lumateperone |
Caplyta |
21
mg-42 mg |
Another antipsychotic medication, Clozaril (brand name) or clozapine
(generic) is an important medication that can often successfully treat those
people who have not responded to more traditional mood stabilizers and who have
persistent suicidal thoughts. The typical adult daily doses for Clozaril are
300-900 mg.
These first-generation antipsychotic medications, such as Haldol
and Thorazine, have significant side effects (e.g., dry mouth, constipation,
sedation, seizures, excessive salivation, blurred vision, nausea, heartburn,
and weight gain) and have been associated with a serious blood disorder
(agranulocytosis, which causes soreness of the mouth, throat, and gums and a
high fever). Thus, it is never considered a first-line medication choice.
However, despite the problematic side effects, we do use them for agitation
management.
Please note that all of the following information regarding
antipsychotics pertains to the atypical antipsychotics or
SGAs (not Clozaril).
Uses:
Treat mania and agitation, mixed features, rapid cycling, hypomania and
depression.
Onset of Effects:
Antipsychotic medications are used to treat mania and can begin to reduce
severe agitation within a few hours to a few days, however, the reduction of
more pronounced manic symptoms is similar to that seen with other mood
stabilizers such as lithium and anticonvulsants (7-10 days or longer).
Laboratory Tests:
All SGAs can cause
metabolic syndrome, so we need periodic measures of blood glucose,
triglycerides, cholesterol, weight, and body mass index (BMI). These should be
done at the beginning of treatment, every three to six months, and yearly
afterward.
Common Side Effects for SGAs or Atypical Antipsychotics:
- Drowsiness and
lethargy (can occur with all, except Abilify)
- Weight Gain (can occur
with all, however minimal with Latuda, Vraylar and Caplyta)
- Nausea, vomiting, heartburn
Less Common Side Effects (these should be reported to prescribing physician):
- Increased risk of
hyperglycemia and type II diabetes (primarily with Clozaril and Zyprexa … less
common with Geodon and Abilify; moderate risk with Risperdal and Seroquel)
- Increased risk of
elevated LDL cholesterol and triglycerides (more common with Clozaril and
Zyprexa … less common with Geodon and Abilify; moderate risk with Risperdal and
Seroquel)
- Breast tenderness,
liquid discharge from breasts (can occur with high doses of Risperdal)
- Severe sunburn when
exposed to even moderate amounts of sunlight
- Abnormal, involuntary
movements of the mouth, tongue, and sometimes head, neck and hands (rare). These
are called tardive dyskinesia (TD).
Rare or Potentially Dangerous Side Effects (if these occur, patient
should immediately contact prescribing physician):
- Soreness in mouth,
throat, and gums
- Moderate to severe
nausea, vomiting, and flu-like symptoms
- Yellow tint to skin or
eyes
- High fever (especially
if accompanied by muscle stiffness)
- Unusual bruising or
bleeding
- Frequent urination or
loss of bladder control
Habit-forming / Addiction Potential:
None
Interactions with other medications (varies depending on the specific drug):
- Levodopa: decreases
effectiveness of levodopa
- Anticonvulsants: can
decrease antipsychotic blood levels
- Digoxin: may increase
blood levels of digoxin and the antipsychotic
- Warfarin: may increase
Warfarin blood levels
- Antacids: may
interfere with the absorption of the drug
Safety during pregnancy:
SGAs (atypical antipsychotics) are generally considered to be cautiously used
during pregnancy.
Breast-Feeding:
Antipsychotic medications are secreted in breast milk. Since these are recently
developed medications, there is inadequate information regarding safety for
infants.
Special Concerns:
Avoid exposure to
extreme heat (e.g., saunas)
Grapefruit juice may
interfere with the effects of the drug
Excessive cigarette
smoking may interfere with the effects of the drug
Avoid direct exposure
to sunlight: antipsychotic medications can increase the likelihood of severe
sunburns
Antidepressant Medications
Note: Not recommended in the treatment of bipolar disorder. The
following list is offered simply as a general reference. They are selective
serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake
inhibitors (SNRIs), norepinephrine and dopamine reuptake inhibitors (NDRIs), serotonin
antagonist and reuptake inhibitor (SARI), noradnergic and specific serotonergic
antidepressant (NaSSA) and serotonin modulators.
New Generation Antidepressants: Generic and Brand Names and Typical
Adult Daily Doses
Generic Name |
Brand Name |
Typical Adult Daily Dose |
trazodone |
Desyrel |
50-400 mg |
fluoxetine |
Prozac |
20-80 mg |
bupropion |
Wellbutrin XL/SR |
100-450 mg |
sertraline |
Zoloft |
50-200 mg |
paroxetine |
Paxil, Paxil CR |
20-50 mg |
venlafaxine |
Effexor, Effexor XR |
75-225 mg |
nefazadone |
Serzone |
100-600 mg |
fluvoxamine |
Luvox |
50-300 mg |
mirtazapine |
Remeron |
15-45 mg |
citalopram |
Celexa |
10-40 mg |
escitalopram |
Lexapro |
5-20 mg |
duloxetine |
Cymbalta |
20-80 mg |
desvenlafaxine |
Pristiq |
50-400 mg |
vilazodone |
Viibryd |
10-40
mg |
vortioxetine |
Trintellix |
5-20 mg |
levomilnacipran |
Fetzima |
40-120
mg |
Antidepressants are effective in treating depression, anxiety,
panic attacks, and obsessive-compulsive disorder (OCD).
Uses:
Generalized anxiety disorder, panic disorder, obsessive-compulsive disorder
(OCD), and major depressive disorder.
Onset of Effects:
Generally two to six weeks.
Laboratory Tests:
Not required.
Common Side Effects:
- Energized or anxious
feelings (it occurs with initial high dosage, typically subsides within one to
two weeks)
- Sedation (primarily
with Remeron and Desyrel)
- Difficulty falling
asleep (often subsides in a few weeks)
- Sexual dysfunction:
primarily anorgasmia (difficulty achieving an orgasm despite adequate arousal)
(can occur in 14%-30% of people treated with antidepressants. One exception:
very rare with Wellbutrin).
- Weight gain (primarily
with Remeron; with other antidepressants, weight gain can occur in up to 10% of
people; however, the weight gain typically does not occur until the person has
been taking the drug for a period of time longer than six months)
Rare Side Effects (if these occur, patient should immediately contact
prescribing physician):
- Soreness of mouth,
throat, or gums
- Unusual bruising or
bleeding
- Severe nausea,
vomiting, and flu-like symptoms
- Severe agitation or
restlessness
- Yellow tinge to skin
or eyes; dark colored urine
- Rapid shift into mania
or hypomania; racing thoughts
Habit-forming / Addiction Potential:
None
Interactions with Other Medications (vary depending on the drug):
Do not take with St.
John’s Wort, 5-HTP (dietary supplement), or MAOIs, cimetidine (Tagamet) without
medical supervision.
Safety During Pregnancy:
Most experts agree that some new generation antidepressants are safe for use
during pregnancy (e.g., Prozac, Zoloft, Wellbutrin). Note: the following
antidepressants have only recently come to market and there is inadequate data
to evaluate safety during pregnancy: Cymbalta, Strattera, Lexapro, Celexa,
Serzone, Pristiq, and Remeron. High doses of Desyrel should not be used during
pregnancy. In addition, recent data suggests that Paxil is less safe for use
during pregnancy.
Breast-Feeding:
Antidepressants are secreted in breast milk, but the amounts are extremely low.
Most experts agree that it is safe to breast feed while taking new generation
antidepressants, however we need to use it with precaution.
Special Concerns:
If someone has been taking antidepressants for a period of six weeks or more
and abruptly stops taking the medication, there can be withdrawal symptoms
(this can occur with any of the antidepressants with the exception of Prozac
due to its long half-life). Withdrawal symptoms include nausea, stomach upset,
nervousness, flu-like symptoms, and a peculiar sensation described by patients
as electrical shocks going through one’s head or extremities. Withdrawal
symptoms are very unlikely if one has been taking the medication for less than
six weeks. Moreover, withdrawal symptoms can be avoided almost 100% of the time
by reducing the dose gradually over a period of several weeks.
Combination Drug: Symbyax (Prozac and Zyprexa):
FDA-approved for the treatment of bipolar depression. Common side effects are
those seen with other SSRIs and Zyprexa (e.g., weight gain, increased risk of
type II diabetes, hyperglycemia, and elevations in LDL cholesterol and
triglycerides).
Antianxiety Medications: Benzodiazepines
Benzodiazepine Facts:
Benzodiazepines are also commonly referred to as minor tranquilizers or anti-anxiety
medications or anxiolytics.
Benzodiazepines: Generic and Brand Names and Typical Adult Daily
Doses
Generic Name |
Brand Name |
Typical Adult Daily Dose |
diazepam |
Valium |
5-10 mg |
clonazepam |
Klonopin |
0.5-4.0 mg |
lorazepam |
Ativan |
2-10 mg |
Note: Alprazolam
(Xanax) can provoke mania in bipolar patients.
Benzodiazepine-like Z drugs Used for Sleep: Typical Adult
Nighttime Doses
Generic Name |
Brand Name |
Typical Adult Daily Dose |
zolpidem |
Ambien |
5-10 mg |
zaleplon |
Sonata |
5-10 mg |
eszopiclone |
Lunesta |
1-3
mg |
Note: There
are two non-benzodiazepine prescription sleeping pills, suvorexant (Belsomra,
10 mg-20 mg) and ramelteon (Rozerem, 8 mg).
Uses:
Ttreats acute anxiety and insomnia during episodes of mania. Also used as
adjunct treatment to treat anxiety disorders (such as panic disorder, social
anxiety, and generalized anxiety disorder). In the treatment of mania,
benzodiazepines are generally used only for the first few days of treatment to
reduce agitation; only rarely are these drugs used beyond a couple of weeks.
Onset of Effects:
30-60 minutes.
Laboratory Tests:
None required
Common Side Effects:
Less Common Side Effects:
- Loss of balance and
falls
Habit-Forming / Addiction Potential:
Significant risk for people with a prior personal or family history of
alcoholism or other forms of serious drug abuse. Rozerem has a less habit-forming
character.
Interactions with Other Medications:
When taking benzodiazepines, any other type of medication that causes
drowsiness or impaired alertness and reaction time can be potentially
dangerous, especially if one has to drive an automobile. In addition, alcohol
should not be consumed when taking benzodiazepines.
Safety During Pregnancy:
Benzodiazepines typically are not to be used during pregnancy.
Breast-Feeding:
Benzodiazepines are secreted in breast milk and should not be used when
breast-feeding.
Special Concerns:
If benzodiazepines are being taken on a regular basis, the body develops a
tolerance for the medication. When this happens, the drugs continue to work to
reduce anxiety, but the problem when there is tolerance is that sudden
cessation of the medication can lead to withdrawal symptoms. Withdrawal
symptoms usually include nervousness, agitation, difficulty falling to sleep,
and on occasion can produce seizures. This needs to be taken very seriously. If
a person has been taking a benzodiazepine on a daily basis for more than six
weeks, and especially if the dose is moderate to high, withdrawal reactions are
a very real risk. The patient should never abruptly stop taking the medication
without first consulting with his/her doctor.
Appendix III
Miscellaneous
Omega-3 Fatty Acids
During the past decades, a number of studies have been conducted
with people suffering from bipolar disorder and also major depression.
Preliminary findings strongly suggest that adding omega-3 fatty acids to the
diet can have a positive effect on reducing unipolar depressive symptoms when
added to antidepressant medications in some individuals. Results in bipolar
disorder have been minimal and disappointing. The best source of omega-3 fatty
acids is fish oil because it has better bioavailability in the brain versus
omega-3 from nut and seed oil.
Uses and General Considerations:
Omega-3 Fatty Acids
Studies have found that people treated with omega-3 fatty acids
must take these dietary supplements on a daily basis and over a prolonged
period of time (i.e., building this into one's ongoing diet). Doses recommended
for treating depression are 1000-2000 mg per day. The version of omega-3 that
has effects on mood is EPA.
Older Generation Antipsychotics
As noted above, newer generation antipsychotics have been
developed during the past 40 years. The newer drugs are considerably safer and
have significantly fewer side effects than older-generation antipsychotic
medications. We are simply mentioning these medications here just as a point of
information since in rare instances some people may be treated with these drugs
(brand names): Thorazine, Mellaril, Serentil, Trilafon, Loxitane, Stelazine,
Prolixin, Navane, Orap, and Haldol. Of these, the most common drugs that are
still used these days are Haldol (often useful to initially treat very severe
agitation seen in some types of mania or schizophrenia) and Trilafon.
Anticholinergic Medications
This class of medications is used occasionally to combat side
effects of some antipsychotic drugs (side effects such as: muscle rigidity or
spasms, restlessness, tremor). Again, we will only list these medications
(brand names): Cogentin and Artane. Anticholinergic drugs have their own set of
side effects including: constipation, blurred vision, dry mouth, difficulty
beginning urination, and occasional memory loss, confusion, and delirium.
SAM-e
SAM-e (S-adenosylmethionine) is a naturally occurring bio-molecule found in most living cells. It is felt to be necessary for carrying out a number of important intracellular chemical reactions. SAM-e has been used in Europe for more than 20 years as a treatment for depression. A number of studies have shown it to be equally effective when compared to prescription antidepressants, other studies do not show any antidepression benefits. Most notable is the virtual lack of side effects. It has not been well supported in studies of the treatment of bipolar disorder, however, it may be used as a viable option for treating bipolar depression. However, it is not useful for treating mania and has, in fact, been found to switch people with bipolar depression into states of mania. Another interesting fact is that SAM-e can help reduce some ADHD symptoms. Doses for the treatment of depression range from 400 mg to 1600 mg per day, although recent investigations indicate that often, higher doses (1000-1600 mg per day) may be necessary for effectively reducing depressive symptoms. SAM-e is available over the counter.If you want to try it, you can discuss it with your physician.
St. John’s Wort
St. John’s Wort is an over-the-counter dietary supplement that has been found to have antidepressant properties. A meta-analysis (Linde, et al., 2008) has shown that St. John’s Wort is equally effective to prescription antidepressants in the treatment of depression if taken in large doses (e.g., 1800 mg per day). This herbal remedy is generally well tolerated with few if any side effects. There are reported cases of possible infertility problems associated with its use, although it is as yet unclear whether this is a common side effect. St. John’s Wort requires daily dosing of 600 mg (mild depression) to 1800 mg per day (taken in three divided doses, for major depression), and typically the first signs of symptom improvement take about six weeks to emerge. Thus, the onset of action is longer than that seen with prescription antidepressants. Research on its use in the treatment of bipolar depression is sorely lacking. And as with any other treatment that has antidepressant properties, St. John’s Wort can potentially provoke mania. Caution: although St. John’s Wort, when it is the only medication being taken, appears to be quite safe, it has been found to cause very significant drug-drug interactions. It is strongly advised that patients never take St. John’s Wort without first consulting with their doctor. Given its tendency to cause multiple and complex interactions with a host of medications, it is recommended that it not be used by people being treated with bipolar medications.
Appendix IV
Caffeine Questionnaire
Following is a caffeine questionnaire that allows clinicians to
quickly determine caffeine intake for their clients. Even small amounts of
caffeine exacerbate anxiety symptoms and the ideal caffeine levels for those
with situational stress and anxiety disorders is zero. Small amounts of
caffeine taken in the morning may actually be helpful for people suffering from
depression (if not otherwise contraindicated due to medical conditions).
Everyone knows that caffeine ingested close to bedtime may
interfere with the ability to go to sleep (i.e., initial insomnia). However,
the more problematic effect of caffeine is its tendency to disrupt deep
sleep (also known as slow wave or restorative sleep). High
amounts taken in the morning (e.g., 750 mg+) and modest amounts of caffeine
taken after noon may interfere with the ability to get adequate deep sleep,
even if not causing initial insomnia. Caffeine use among people suffering from
almost any type of psychiatric disorder often exceeds levels that are
acceptable. Preserving deep sleep is essential for emotional and cognitive
functioning. Our patients may not realize or appreciate the negative impact of
excessive caffeine use. Sleep is essential for normal brain functioning and
needs to be optimized in each and every client we see for treatment.
It is important to tell all clients about the need for good
quality sleep and keeping caffeine at acceptable levels is a high yield
intervention. Yet, many of our clients may minimize the importance of this, so
good patient education is important. Generally speaking, for those with
significant psychological disorders, caffeine levels should not exceed 250 mg
per day and should not occur after noon (in order to preserve deep sleep).
Afternoon fatigue is often easily addressed by a ten-minute brisk walk, which
generally results in 60-120 minutes of energy and relief from fatigue. Daily
use of caffeine exceeding 750 mg will require gradual caffeine reduction
to avoid caffeine withdrawal (e.g., reducing daily use by 125 mg per day per
week).
Please feel free to make copies of the following caffeine
questionnaire for use with your patients. Click here for the printable Caffeine Consumption Questionnaire.
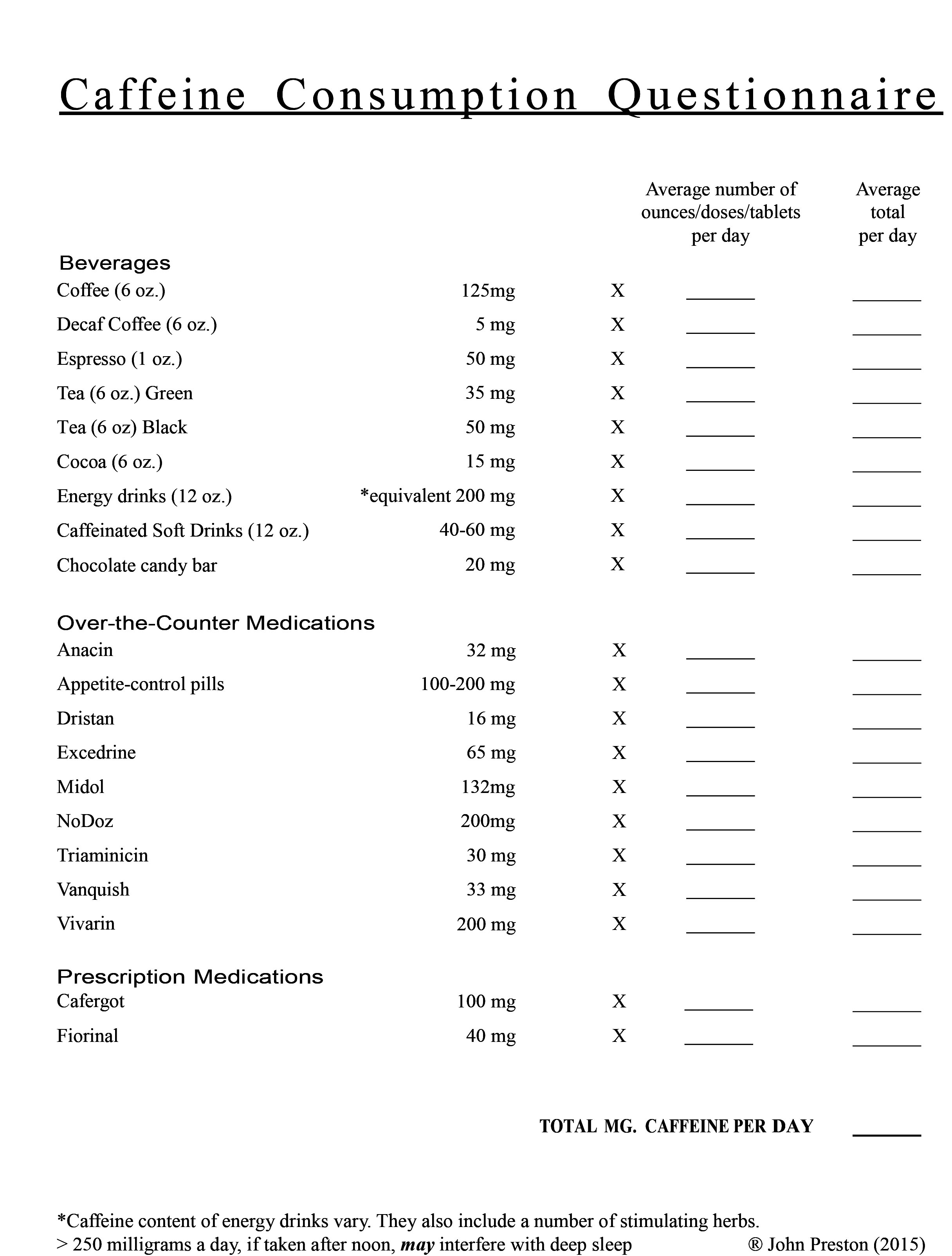
Suggested Readings/Resources
Advoka, C, (2018). Julien’s Primer of Drug Actions. Worth Publishers.
American Medical Association website: (https://www.ama-assn.org/system/files/bhi-psychopharmacology-how-to-guide.pdf)
American Psychiatric Association (2022). DSM-5-TR. Washington, DC.
Carlat Psychiatry Reports: a monthly subscription. Reports for adult psychiatry and one for children and adolescents. PO Box 626, Newburyport, MA
Geller, B. & DelBello, M.P. (Eds.) (2008) Treatment of bipolar disorder in Children and Adolescents. The Guilford Press.
NIH omega-3 fatty acids: health professional fact sheet. https://ods.od.nih.gov/pdf/factsheets/Omega3FattyAcids-Consumer.pdf
Preston, J.D., O'Neal, J. and Talaga, M. (2017) Handbook of clinical psychopharmacology for therapists: Eighth Edition. New Harbinger.
Preston, J.D., O’Neal, J., and Talaga, M. (2015) Childhood and Adolescent Psychopharmacology Made S. Third Edition. New Harbinger.
Preston, J.D., O’Neal, J, and Talaga, M. (2009) Consumer’s guide to psychiatric drugs. Second edition: Penguin Books.
Psychceu.com: (https://www.psychceu.com/quick_reference_bw.pdf)
Stahl, S. (2017). Prescriber’s guide 6th edition, Cambridge University Press.
Texas Health and Human Service: (https://www.hhs.texas.gov/sites/default/files/documents/doing-business-with-hhs/provider-portal/facilities-regulation/psychiatric/psychiatric-drug-formulary.pdf)
DSM-5-TR by American Psychiatric Association, 2022.
References
Alarcon, R. D., Glover, S., Boyer, W., & Balon, R. (2000). Proposing an Algorithm for the Pharmacological Management of PTSD. Annals of Clinical Psychiatry, 12(4), 239-246.
American Medical Association (n.d.). Psychopharmacology How-to Guide. https://www.ama-assn.org/system/files/bhi-psychopharmacology-how-to-guide.pdf
Arborelius, L., Owens, M. J., Plotsky, P. M., & Nemeroff, C. B. (1999). The Role of Corticotropin Releasing Factor in Depression and Anxiety Disorders. Journal of Endocrinology, 166, 1-12.
Barkley, R. A. (2000). Taking Charge of ADHD. Guilford Press.
Bauer, M., Alda, M., Priller, J,, & Young, L T. (2003). Implications of the Neuroprotective Effects of Lithium for the Treatment of Bipolar and Neurodegenerative Disorders. Pharmacopsychiatry, 36(3), S250-254. https://doi.10.1055/s-2003-45138
Benes, F. M., Vincent, S. L., & Todtenkopf, M. (2001). The Density of Pyramidal and Non-pyramidal Neurons in Anterior Cingulate Cortex of Schizophrenic and Bipolar Subjects. Biological Psychiatry, 50(6), 395-406.
Biederman, J., Wilens, T., Mick E., Spencer, T., & Faraone, S. V. (1999). Pharmacotherapy for ADHD Reduces Risk for Substance Abuse Disorder. Pediatrics, 104(2), e20.
Blehar, M. C. & Rosenthal, N. E. (1989). Seasonal Affective Disorders and Phototherapy. Archives of General Psychiatry, 46(5), 469-474.
Bowden, C. L., Calabrese, J. R., Sachs, G. S., Calabrese, J. R., Sachs, G., Yatham, L. N., Asghar, S. A., Hompland, M., Montgomery, P., Earl, N., Smoot, T. M., & DeVeaugh-Geiss, J. (2004). A Placebo-controlled 18-month Study of Lamotrigine and Lithium Maintenance Treatment in Recently Depressed Patients with Bipolar I Disorder. Archives of General Psychiatry.
Calabrese, J. R., Bowden, C. L., Sachs, G. S., Ascher, J. A., Monaghan, E., & Rudd, G. D. (1999). A Double-Blind, Placebo-Controlled Study of Lamotrigine Monotherapy in Outpatients with Bipolar I Depression. Journal of Clinical Psychiatry, 60, 78-88.
Castellanos, F. X., Lee, P. P., Sharp. W., Jeffries, N. O., Greenstein, D. K., Clasen, L. S., Blumenthal, J. D., James, R. S., Ebens, C. L., Walter, J. M., Zijdenbos, A., Evans, A. C., Giedd, J. N., & Rapoport, J. L. (2002). Developmental Trajectories of Brain Volume Abnormalities in Children and Adolescents with ADHD. Journal of the American Medical Association, 288(14), 1740-1748.
Cooper, J. R., Bloom, F. E., & Roth, R. H. (2003). The Biochemical Basis of Neuropharmacology. Oxford University Press.
Davis, L. L., English B. A., Ambrose, S. M., & Petty, F. (2001). Pharmacotherapy for PTSD: A Comprehensive Review. Expert Opinion on Pharmacotherapy, 2(10), 1583-1595.
Dawson, R., Lavori, P. W., Coryell, W. H., Endicott, J., & Keller, M. B. (1999). Course of Treatment Received by Depressed Patients. Journal of Psychiatric Research, 33, 233-244.
DeBattista, C, & Schatzberg, A. F. (2002) Current Psychotropic Dosing and Monitoring Guidelines. Primary Psychiatry, 9(6), 34-48.
Delgado, P. L., Charney, D. S., Price, L. H., Aghajanian, G. K., Landis, H., & Heninger, G. R. (1990). Serotonin Function and the Mechanism of Antidepressant Action: Reversal of Antidepressant Induced Remission by Rapid Depletion of Plasma Tryptophan. Archives of General Psychiatry, 47, 411-418.
Delle Chiaie, R., Pancheri, P., & Scapicchio, P. (2002). Efficacy and Tolerability of Oral and IM SAM-E in the Treatment of Major Depression: Comparison with Imipramine in 2 Multi-Center Studies. American Journal of Clinical Nutrition, 76, 1172S-1176S.
Doraiswamy, P. M., Kahn, Z. M., Donahue, R. M., & Richard, N. E., (2001). Quality of Life in Geriatric Depression: A Comparison of Remitters, Partial Responders, and Nonresponders. American Journal of Geriatric Psychiatry, 9, 4223-428.
Docherty, J. P. (1997). Barriers to the Diagnosis of Depression in Primary Care. Journal of Clinical Psychiatry, 58(Suppl. 1), 5-10.
Drake, C., &, Roehrs, T., Shambroom, J., & Roth, T. (2013). Caffeine Effects on Sleep Taken 0, 3, or 6 Hours before Going to Bed Journal of Clinical Sleep Medicine, 9(11). https://doi.org/10.5664/jcsm.3170
Dubovsky, S. L. (2001). New Concepts in the Treatment of Mania. U.C. Davis Symposium.
Echols, J. C. Naidoo, U., & Salzman, C. (2000). SAM-e (S-adenosylmethionine). Harvard Review of Psychiatry, 8, 84-90. https://doi/10.1080/hrp_8.2.84
Fava, M. (2000). New Approaches to the Treatment of Refractory Depression. Journal of Clinical Psychiatry, 61(Suppl. 1), 26-32.
Frank, E., Hlastala, S., Ritenour, A., Houck, P., Tu, X. M., Monk, T. H., Mallinger, A. G., & Kupfer, D. J. (1997). Inducing Lifestyle Regularity in Recovering Bipolar Disorder Patients. Biological Psychiatry, 41, 1165-1173.
Geller, B. & DelBello, M. P. (Eds.) (2003). Bipolar Disorder in Childhood and Early Adolescence. The Guilford Press.
George, M. S. (2003). Stimulating the Brain. Scientific American, 289(3), 66-73.
Ghaemi, S. N., Lenox, M. S., & Baldessarini, R. J. (2001). Effectiveness and Safety of Long-term Antidepressant Treatment in Bipolar Disorder. Journal of Clinical Psychiatry, 62(7), 565-569. doi: 10.4088/jcp.v62n07a12
Gitlin, M. (2002). Recognition and Resolve: Treatment Resistant Depression. U.S. Psychiatric Congress.
Gitlin, M. (2002). Depression Myths and Contrary Realities. Continuing Education Series, UCLA.
Goodwin, F. K., & Jamison, K. R. (2007). Manic-Depressive Illness (2nd ed.) . Oxford University Press.
Heim, C. & Nemeroff, C. P. (2002.) Neurobiology of Early Life Stress. Seminars in Clinical Neuropsychiatry, 7(2). 147-159.
Hirschfeld, R. M. A., Bowden, C. L., Gitlin, M. J., Keck, P. E., Perlis, R. H., Suppes, T., Thase, M. E. & Wagner, K. D. (2002). Practice Guidelines for Patients with Bipolar Disorder. American Journal of Psychiatry, 159, 1-50.
Hirschfield, R. M. A. (2001). Bipolar Spectrum Disorders. Journal of Clinical Psychiatry, 62(Suppl. 14), 5-9.
Judd, L. L., Akiskal, H. S., Schettler, P. J., Endicott, J., Maser, J., Solomon, D. S., Leon, A. C., Rice, J. A., & Keller, M. B. (2002). The Long Term Natural History of the Weekly Symptomatic Status of Bipolar I Disorder. Archives of Psychiatry, 59, 530-537. doi:10.1001/archpsyc.59.6.530
Katz, S. J., Kessler, R. C., Lin, E., & Wells, K. B. (1998). Medication Management of Depression in the U.S. and Ontario. Journal of General Internal Medicine, 13, 77-85.
Kempermann G., & Gage, F. H. (1999). New Nerve Cells for the Adult Brain. Scientific American, 280(5), 48-53.
Kessler, R. C., McGonagle, K. A., Zhao, S., Nelson, C. B., Hughes, M., Eshleman, S., Wittchen, H., & Kendler, K. S. (1994). Lifetime and 12-Month Prevalence of DSM-IV Psychiatric Disorders in the United States. Results from the National Comorbidity Survey. Archives of General Psychiatry, 151(1), 8-19. https://doi.org/10.1001/archpsyc.1994.03950010008002
Kuczenski, R. & Segal, D. S. (2002). Exposure of Adolescent Rats to Oral Methylphenidate. Journal of Neuroscience, 22, 264-271.
Lawlor, D. A., & Hopker, S. W. (2001). The Effectiveness of Exercise as an Intervention in the Management of Depression: Systematic Review and Meta-Regression Analysis of Randomized Controlled Trials. Bristol Medical Journal, 322(7289), 763-767. Department of Social Medicine, University of Bristol. doi: 10.1136/bmj.322.7289.763
LeDoux, J. (2016) Anxious. Penguin.
Linde, K., Berner, M. M., & Kriston, L (2008). St. John'’s Wort for Depression. Cochrane Database of Systematic Reviews, 4(CD000448). https://doi.org/10.1002/14651858.CD000448.pub3
Lu, S. C. (2000). S-Adenosylmethionine. International Journal of Biochemistry and Cell Biology, 32, 391-395. doi: 10.1016/s1357-2725(99)00139-9
Malkoff-Schwartz, S., Frank, E., Anderson, B., Sherrill, J. T, Siegel, L., Patterson, D., & Kupfer, D. J. (1998). Stressful Life Events and Social Rhythm Disruption in the Onset of Manic Depressive Bipolar Episodes: A Preliminary Investigation. Archives of General Psychiatry, 55, 702-707.
Metzner, R. J. (2000). Neurotransmitters and Depression: New Possibilities. Annual Meeting.American Psychiatric Association.
MTA Cooperative Group (1999). A 14-month randomized Randomized Clinical Trial of Treatment Strategies for ADHD. Archives of General Psychiatry, 56(12), 1073-1086. doi: 10.1001/archpsyc.56.12.1073 National Institutes of Health. On Omega and Other Supplement Information.
Nestler, E., Kenny, P. J., Russo, S. J., & Schaefer, A. (2020). Molecular Neuropharmacology (4th ed.). McGraw-Hill. (Original work published 2001)
Paykel, E. S., Ramana, R., Cooper, Z., Hayhurst, H., Kerr, J., & Barocka, A. (1995). Residual Symptoms after Partial Remission: An Important Outcome in Depression. Psychological Medicine, 25, 1171-1180. doi: 10.1017/s0033291700033146
Peet, M. & Horrobin, D. F. (2002). A Dose Ranging Study of the Effects of Ethyl-Eicosapentaenoate in Patients with Ongoing Depression Despite Apparently Adequate Treatment with Standard Drugs. Archives of General Psychiatry, 59, 913-919. doi: 10.1001/archpsyc.59.10.913
Phelps, J. (2016). A Spectrum Approach to Mood Disorders. WW Norton.
Pope, M, & Scott, J. (2003). Do Clinicians Understand Why Individuals Stop Taking Lithium? Journal of Affective Disorders, 74, 287-291. doi: 10.1016/s0165-0327(02)00341-5
Power, M. (Ed.) (2004). Mood Disorders: A Handbook of Science and Practice. John Wiley and Sons.
Preston, J. D. (2006). Integrative Brief Therapy (2nd ed.). Impact Publishers.
Preston, J. D., O’Neal, J. H., & Talaga, M. C. (2017). Handbook of Clinical Psychopharmacology for Therapists (8th ed.). New Harbinger.
Psychceu.com: (https://www.psychceu.com/quick_reference_bw.pdf)
R, McQuaid, R., McInnis, O. A., Abizaid, A., & Anisman, H.. (2014). Making room for Oxytocin in Understanding Depression. Neuroscience and Biobehavioral Reviews. https://doi.org/10.1016/j.neubiorev.2014.07.005
Reynolds, C. F., Frank, E., Perel, J. M., Imber, S. D., Cornes, C., Miller, M. D., Mazunder, S., Houck, P. R., Dew, M. A., Stack, J. A., Pollock, B. G., & Kupfer, D. J. (1999). Nortriptyline and Interpersonal Psychotherapy as Maintenance Therapies for Recurrent Major Depression: A Randomized Controlled Trial in Patients Older than 59 Years. JAMA, 281, 39-45.
Riddle, S. (2001). Drug Interactions: Examining the Impact of Botanicals and Dietary Supplements. Future Dimensions in Clinical Nutrition Management, 20(3), 7-14.
Rosenthal, N, E. (2013). Winter Blues Survival Guide. Guilford Press
Rush J., Trivedi, H., Trivedi, M. H., Wisniewski, S. R., Nierenberg, A. A., Stewart, J. W., Warden, D., Niederehe, G., Thase, M. E., Lavori, P. W., Lebowitz, B. D., McGrath, P. J., Rosenbaum, J. F., Sackeim, H. A., Kupfer, D. J., Luther, J., & Fava M. (2006). Acute and Longer- term Outcomes in Depressed Outpatients Requiring One or Several Treatment Steps: A STAR*D Report. American Journal of Psychiatry, 163,1898-1904. doi: 10.1176/ajp.2006.163.11.1905
Sapolsky, R. M. (2017). Behave. Penguin Random House.
Shelton, R. C. & Tomarken, A. J. (2001). Can Recovery from Depression Be Achieved? Psychiatric Services, 52(11), 1469-1478. doi: doi.org/10.1176/appi.ps.52.11.1469
Spiegel, D. A. & Bruce, T. J. (1997). Benzodiazepines and Exposure-based Cognitive Behavior Therapies for Panic Disorder: Conclusions from Combined Treatment Trials. American Journal of Psychiatry, 154, 773-781.
Stahl, S. (2021). Stahl’s Essential Psychopharmacology. (5th ed.) Cambridge University Press.
Stoll, A. L., Severus, W. E., Freeman, M. P,, Rueter, S., Zboyan, H. A., Diamond, E., Cress, K. K., & Marangell, L. B. (1999). Omega 3 Fatty Acids In Bipolar Disorder. A Preliminary Double-Blind, Placebo-Controlled Trial. Archives of General Psychiatry, 56, 407-412. DOI: 10.1001/archpsyc.56.5.407
Terman, M, Terman, J. S., & Ross, D. C. (1998). A Controlled Trial of Timed Bright Light and Negative Ionization for Treatment of Winter Depression. Archives of General Psychiatry, 55, 875-882. doi: 10.1001/archpsyc.55.10.875
Texas Dept. of Mental Health (1998). Texas Medication Algorithm Project.
Thase, M. E., & Rush, A. J. (1997). When at First You Don’t Succeed: Sequential Strategies for Antidepressant Nonresponders. Journal of Clinical Psychiatry, 58(Suppl. 13), 23-29.
Trivedi, M. H., & Klieber, B. A., (2001) Algorithm for the Treatment of Chronic Depression. Journal of Clinical Psychiatry, 62(Suppl. 6), 22-29.
U.S. FDA (2023) Spilling the Beans: How Much Caffeine is Too Much? FDA.gov.
Zimmerman, M., Posternak, M. A., & Chelminski, I. (2002). Symptom Severity and Exclusion from Antidepressant Efficacy. Journal of Clinical Psychopharmacology, 22(6), 610- 614. doi: 10.1097/00004714-200212000-00011



 ContinuingEdCourses.Net dba SocialWorkCoursesOnline.com, provider #1107, is approved as an ACE provider to offer social work continuing education by the Association of Social Work Boards (ASWB) Approved Continuing Education (ACE) program. Regulatory boards are the final authority on courses accepted for continuing education credit. ACE provider approval period: 3/9/2005-3/9/2027.
ContinuingEdCourses.Net dba SocialWorkCoursesOnline.com, provider #1107, is approved as an ACE provider to offer social work continuing education by the Association of Social Work Boards (ASWB) Approved Continuing Education (ACE) program. Regulatory boards are the final authority on courses accepted for continuing education credit. ACE provider approval period: 3/9/2005-3/9/2027. ContinuingEdCourses.Net dba SocialWorkCoursesOnline.com has been approved by the National Board for Certified Counselors (NBCC) as an Approved Continuing Education Provider (ACEP), ACEP #6323. Programs that do not qualify for NBCC credit are clearly identified. ContinuingEdCourses.Net is solely responsible for all aspects of the programs.
ContinuingEdCourses.Net dba SocialWorkCoursesOnline.com has been approved by the National Board for Certified Counselors (NBCC) as an Approved Continuing Education Provider (ACEP), ACEP #6323. Programs that do not qualify for NBCC credit are clearly identified. ContinuingEdCourses.Net is solely responsible for all aspects of the programs. ContinuingEdCourses.Net dba SocialWorkCoursesOnline.com is recognized by the New York State Education Department's State Board for Social Work (NYSED-SW) as an approved provider of continuing education for licensed social workers #SW-0561.
ContinuingEdCourses.Net dba SocialWorkCoursesOnline.com is recognized by the New York State Education Department's State Board for Social Work (NYSED-SW) as an approved provider of continuing education for licensed social workers #SW-0561.
